Introduction
Materials and Methods
Cells origin and ethical statements
Human adipose stromal cells culture conditions
Flow cytometry analysis
Differentiation capacity test
Cell viability – MTT reduction test
Semi-quantitative electron spin resonance (ESR) analysis of ascorbyl free radical (AFR)
hASCs treatment for analysis of tenogenic differentiation
500 μM AA-2P and 1% ITS (v/v) in GM (named AA- 2P)
Medium with reduced (to 2% v/v) serum content (named 2% FBS)
500 μM AA-2P and 1% ITS (v/v) in medium with reduced serum content (named AA-2P+2% FBS)
Immunocytochemical analysis of SCLERAXIS (SCX) nuclear translocation– time dependent preliminary experiment
Analysis of tenogenesis-related genes expression with the use of qRT-PCR
Analysis of tenogenesis-related proteins synthesis changes with the use of western blot (WB)
Immunocytochemical evaluation (ICC) of collagens expression and semi-quantitative microphotographs analysis
Statistical analysis
Results
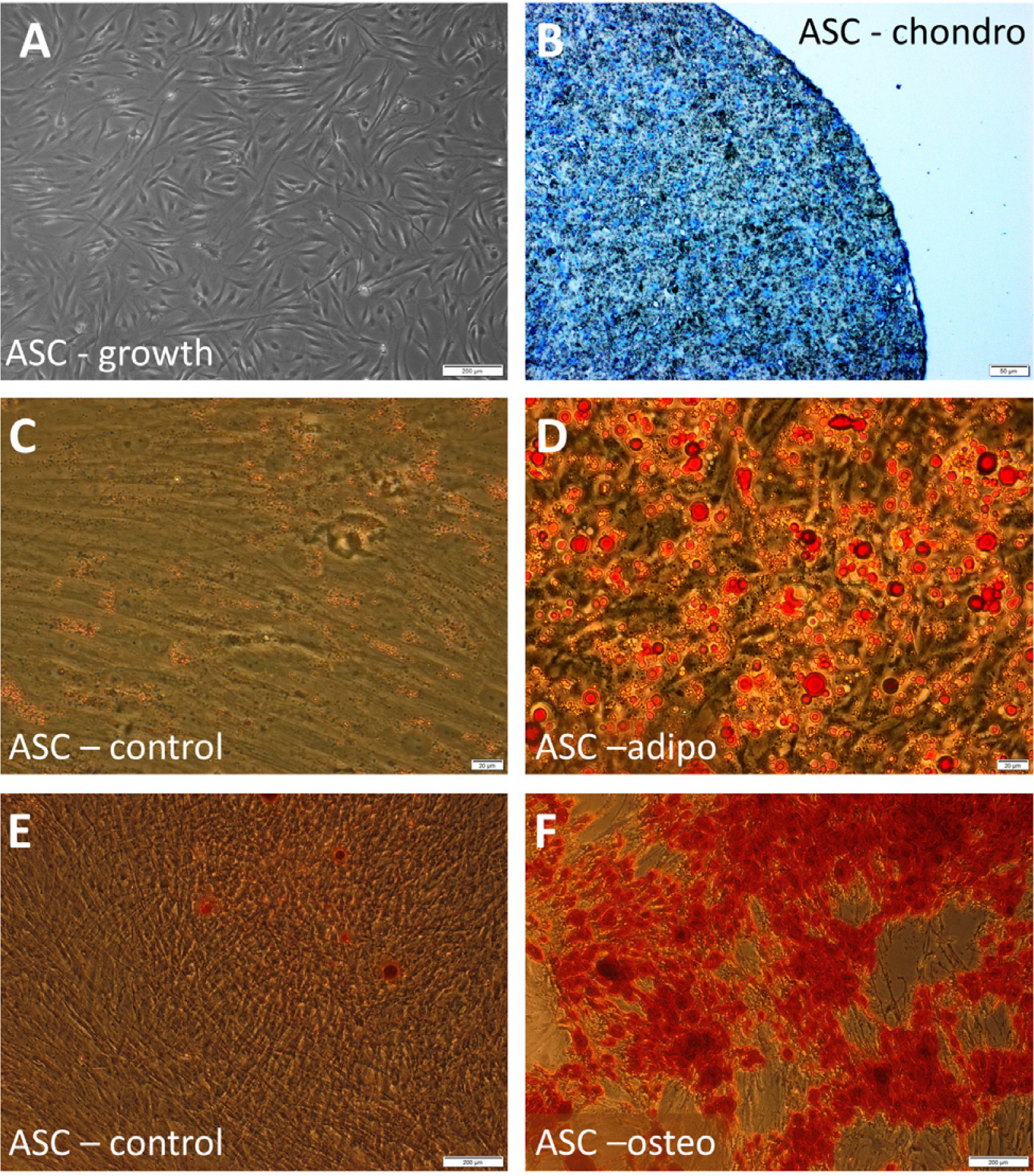 | Fig. 1Morphology and differentiation potential of investigated cells. (A) morphology of untreated hASCs; (B) hASCs after chondrogenic differentiation, chondropellet stained with Toluidin blue; (C, D) Oil Red O staining of hASCs cultured in a standard (C) or adipogenic medium (D) (visible fat droplets stained in red); (E, F) Alizarin Red S staining of hASCs cultured in a standard (E) or osteogenic medium (F) (visible red calcium deposits). Scale bars: 200 μm (A, E, F); 50 μm (B); 20 μm (C, D). |
The effect of AA-2P with or without ITS on hASCs viability
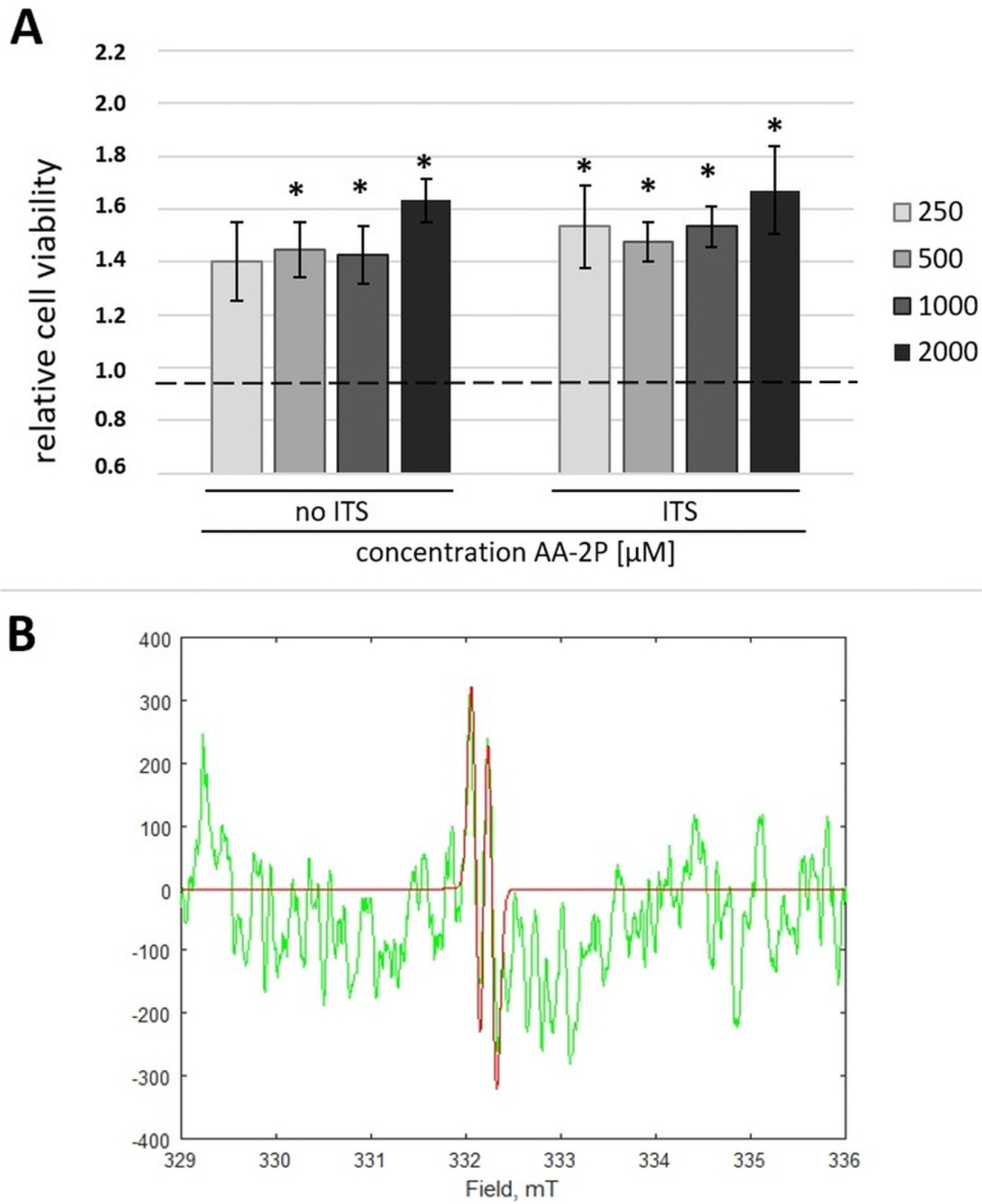 | Fig. 2The effect of ascorbic derivatives on adipose stromal cells (ASCs). (A) MTT test. The effect of L-ascorbic acid-2-phosphate (AA-2P) with or without insulin-transferrin-selenium (ITS) supplementation on hASCs. *p<0.05 in comparison to untreated control (Wilcoxon test); (B) Electron Spin Resonance (ESR). In green – representative experimental ESR spectrum for ascorbyl free radicals (AFR) generated directly from a cell supernatant sample. In red – computer spectral simulation of AFR signal (aH=0.18 mT) enabling quantification of the signal intensity. |
ITS addition does not affect AFR formation
Day 5 selected as the optimal time point for of tenogenic differentiation analysis
 | Fig. 3The dynamics of hASC response to AA-2P treatment with or without reduced serum level evaluated by immunocytochemical method. (A) The mean translocation ratio (MTR) of SCLERAXIS analyzed in hASCs at day 3, 5 and 7 of treatment. The factor calculated as the ratio of mean green fluorescence in the nucleus to the mean green fluorescence in the cytoplasm in certain object (a cell) which indicates activation of the transcription factor in response to experimental treatment; (B) The graphs represent mean green fluorescence in the nuclei, which refers to the nuclear expression of SCLERAXIS. hASCs from 3 donors were used in the experiments. Data analyzed by Student’s t-test for relative samples. *p<0.05 for the comparison of treated cells to control, untreated cells; #p<0.05 the comparison of cells in the same experimental conditions between different time points. Abbreviations: GM - untreated internal control samples cultured in growth medium analyzed in respective time points, AA-2P- ascorbic acid 2-phosphate treatment; 2% FBS- cells cultivated with decreased serum level; AA-2P+2%FBS- treatment with combination of two factors; (C) representative pictures of cells from one donor, 5th day of treatment. Different rows present the same fields of view - cells in various experimental conditions. SCLERAXIS stained in green, nuclei stained in blue. |
Tested agents activate tenogenic pathway in hASCs to a different extent
 | Fig. 4The treatment with AA-2P and/or reduced serum level affects the process that regulates the expression of genes associated with tenogenesis. Gene expression determined by RT-PCR and calculated using the 2ΔΔCt method. Results presented as fold change with 95% confidence interval in relation to the untreated internal control samples (cells from the same donor cultured in parallel in GM) which value was taken as=1. Y axis presented in log scale; Statistical analysis was performed by comparison of ddCt values in 2 step protocol: 1) by one-sample Student’s t-test, comparison of means obtained by subtraction Ct[treated]-Ct[control]; n=6 for each group, *p<0.05; 2) comparison of treatment effect between groups by one way ANOVA for differences [(AA-2P)-CTRL] vs [(2% FBS)-CTRL] vs [ (AA-2P+2% FBS)-CTRL], letters A, B and C above bars indicate statistical differences: groups sharing the same letter do not differ significantly (p<0.017, n=6 for each group); CTRL-untreated control; AA-2P- ascorbic acid 2-phosphate treatment; 2% FBS- cells cultivated with decreased serum level; AA-2P+2%FBS- treatment with combination of two factors; SCX- SCLERAXIS; MKX- MOHAWK HOMEOBOX; COL1- COLLAGEN, type I, alpha 1; COL3- COLLAGEN, type III, alpha 1; DCN- DECORIN. |
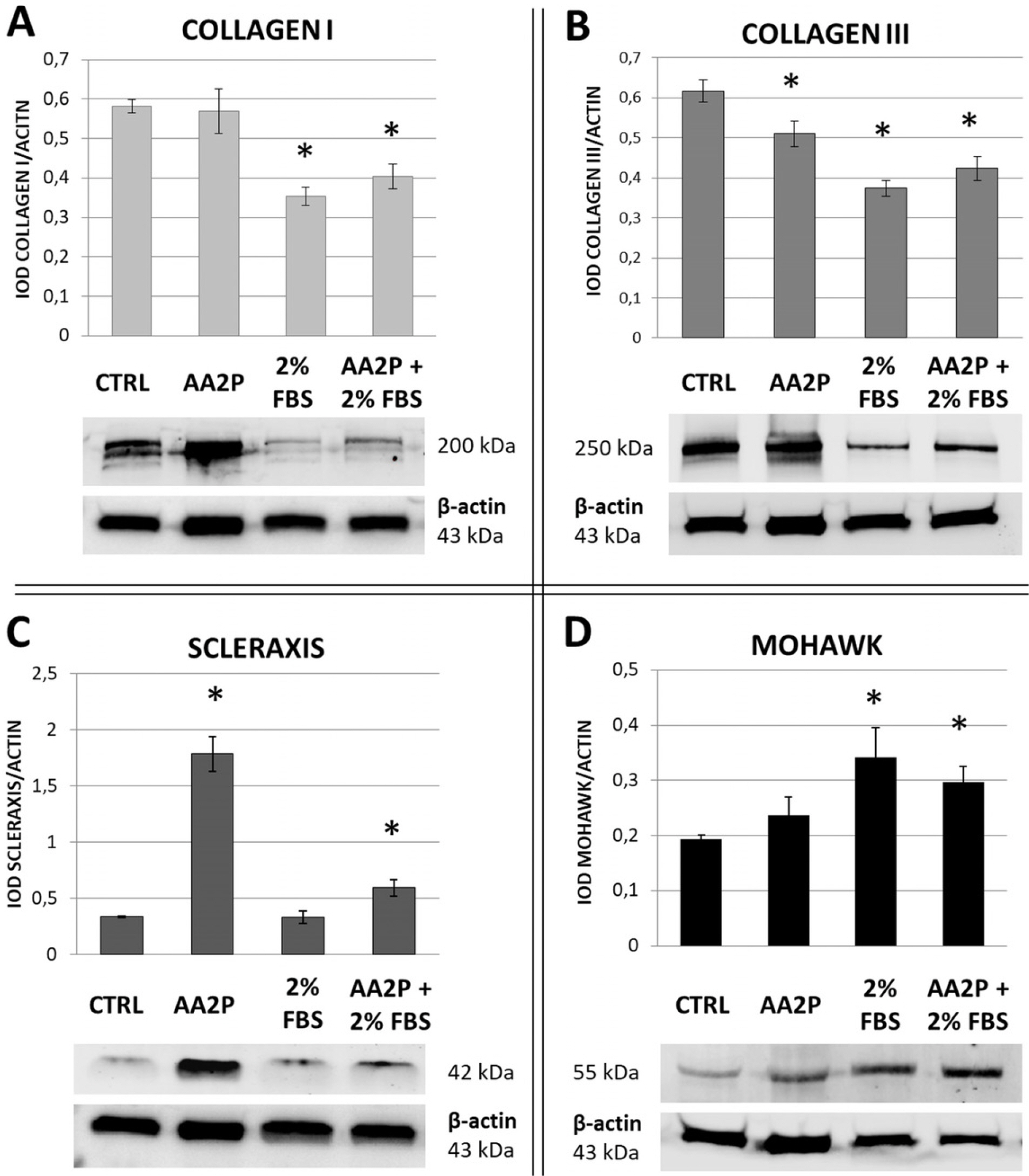 | Fig. 5The effect of serum reduction and ascorbic acid 2-phosphate (AA- 2P) treatment on tenogenesis-related proteins synthesis in adipose stromal cells (ASCs). Western Blot analysis. β-actin was used as a reference protein. hASCs from 3 donors were used in the experiment. At least 3 repetitions were performed for each donor and each treatment. *p<0.05 in comparison to control, untreated cells (Wilcoxon test). CTRL-untreated control; AA-2P- ascorbic acid 2-phosphate treatment; 2% FBS- cells cultivated with decreased serum level; AA-2P+2%FBS- treatment with combination of two factors. |
AA-2P treatment impacts extracellular distribution of collagen and total collagen production
 | Fig. 6Imaging analysis of type I and type III collagen production by adipose stromal cells treated with ascorbic acid 2-phosphate. Immunocytoche-mistry. The synthesis of COLLAGEN I (A) and COLLAGEN III (B) in hASCs cultured in standard medium (upper panels) or in medium supplemented with 500 μM AA-2P+1% ITS (lower panels). Cell nuclei were stained with DAPI. All images in one raw represent the same field of view. White arrows – intracellular collagen, blue arrows – extracellular collagen. Objective 10×, scale bar – 100 μm (applies to images from the figure). |
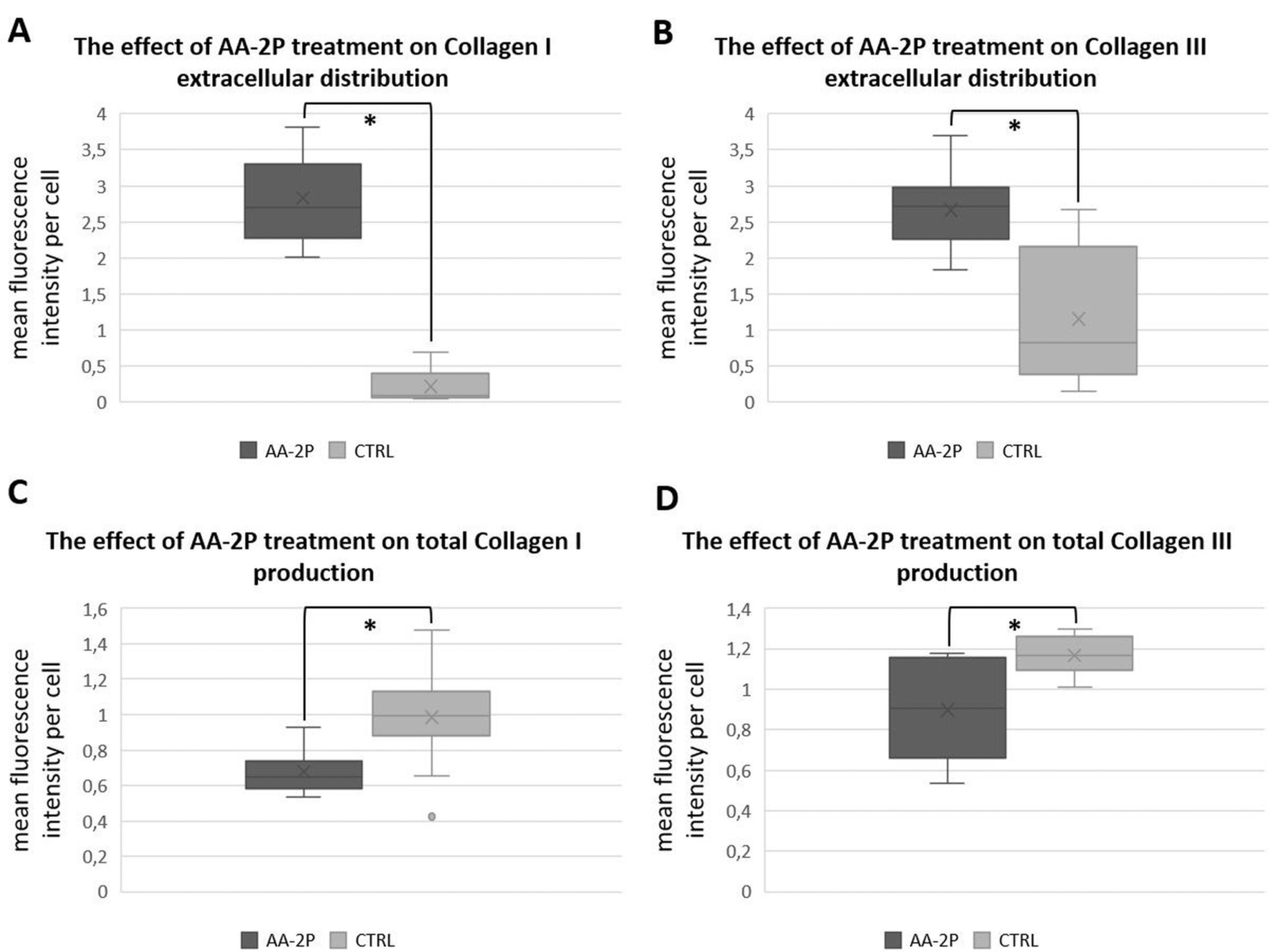 | Fig. 7The effect of ascorbic acid 2-phosphate (AA-2P) on the collagens distribution in adipose stromal cells. Relative quantity of extracellularly distributed COLLAGEN type I (A) and type III (B) and total COLLAGEN type I (C) and type III (D) depending on addition of AA-2P+ITS to culture media. Data presented as a five-number summary (minimum, first quartile, median, third quartile and maximum). “x” – mean value. *p<0.05 (U-Mann-Whitney test). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download