Abstract
Background and Objectives
Mesenchymal stem cells (MSCs) have immense therapeutic potential for treating intractable and immune diseases. They also have applications in regenerative medicine in which distinct treatments do not exist. Thus, MSCs are gaining attention as important raw materials in the field of cell therapy. Importantly, the number of MSCs in the bone marrow is limited and they are present only in small quantities. Therefore, mass production of MSCs through long-term culture is necessary to use them in cell therapy. However, MSCs undergo cellular senescence through repeated passages during mass production. In this study, we explored methods to prolong the limited lifetime of MSCs by culturing them with different seeding densities.
Methods and Results
We observed that in long-term cultures, low-density (LD, 50 cells/cm2) MSCs showed higher population doubling level, leading to greater fold increase, than high-density (HD, 4,000 cells/cm2) MSCs. LD-MSCs suppressed the expression of aging-related genes. We also showed that reactive oxygen species (ROS) were decreased in LD-MSCs compared to that in HD-MSCs. Further, proliferation potential increased when ROS were inhibited in HD-MSCs.
Mesenchymal stem cells (MSCs) have immense potential as therapeutic agents. To use them for cell therapies in the clinical setting, the use of conventional in vitro cultivation methods is essential (1-3). Unfortunately, the in vitro cultivation methods involve continuous passaging, during which MSCs undergo cellular senescence (4). Senescent MSCs have typical characteristics, including flattened morphology and enlarged cellular shape. Moreover, their proliferation abilities and differentiation potentials are significantly decreased; thus, they cannot be expected to perform optimally (5, 6), limiting their clinical applications. To overcome these shortcomings, researchers have explored several strategies to delay the aging processes of MSCs (7-11). Some of these strategies include repairing DNA damage induced by reactive oxygen species (ROS) produced in cultures, improving proteostasis by increasing autophagy activity, restricting caloric intake, and enhancing mitochondrial function using resveratrol (12-14). These results are achieved by regulating the pathways involved in stem cell aging.
High cell density, usually greater than 3,000 cells/cm2, is used to manufacture MSC products for clinical uses. There are a few reports addressing the relationship between cell density and change in stem cell function. In human adipose tissue-derived mesenchymal stem cells (AD-MSCs), cultures with low cell density (200 cells/cm2) reportedly show high proliferation activity and different cell morphologies and sizes (15, 16). Moreover, the expression of stemness-related gene is increased when cell density is low (17). However, the expression of genes related to immunosuppression, migration, and cell motility is upregulated at a high cell density (5,000 cells/cm2) (16).
These reports indicate that cell density has effects on morphological as well as functional characteristics of MSCs. Therefore, cell characteristics and functions can probably be manipulated by changing cell density in vitro. However, no such cell density-dependent changes in the characteristics and aging phenomenon of MSCs have been observed in long-term culture of bone marrow-derived mesenchymal stem cells (BM-MSCs).
In this study, we evaluated whether BM-MSC senescence during continuous passaging can be attenuated by maintaining low cell density. We examined and compared stem cell morphology, proliferation, and aging-dependent characteristics such as ROS production, DNA damage, telomerase activity, aging-dependent gene expression, and staining with β-galactosidase in cultures of varying cell densities at three passage stages. Based on these results, we proved that senescence in late passage stages could be attenuated by culturing at low cell density, indicating that MSC senescence can be delayed and that life span can be extended by controlling cell density in vitro. These results can be used as important data for the mass production of stem cell therapeutic products.
Human bone marrow collection was approved by the Seoul St. Mary’s Hospital Institutional Review Board (IRB), and written informed consent was obtained from a healthy donor (IRB number #KC15CSSE0336). Isolated MSCs were characterized for several cell surface antigens by flow cytometry (FACS Calibur; BD Biosciences). The MSCs were positive for CD29, CD44, CD73, CD90, and CD105, but negative for CD14, CD31, CD104, and HLA- DR.
In this study, MSCs cultured at three different cell densities were used for all experimental analyses: low-density cultured MSCs [LD-MSCs (LD), 50 cells/cm2], middle- density cultured MSCs [MD-MSCs (MD), 1,000 cells/cm2], and high-density cultured MSCs [HD-MSCs (HD), 4,000 cells/cm2]. LD and MD were passaged at intervals of 7∼10 days, whereas HD were passaged every 3∼5 days and cultured for long term until late passage. To observe the effects of ROS scavengers on MSC proliferation, cells were treated with ascorbic acid (AA; 25 μg/ml). Cell prolife-ration was determined by cell counting. Proliferation fold increase (FI), population doubling time (PDT), and population doubling level (PDL) were calculated by counting initial seeding numbers, final cell numbers obtained from each passage, and culture period per passage.
Adipogenic and osteogenic differentiations were performed using the protocol described previously (18). For quantitative analysis, Oil red O-stained cells were completely dried and de-stained using 100% isopropanol. The absorbance of the de-stained solution was measured at 500 nm. Similarly, Alizarin Red S-stained cells were completely dried, de-stained with 10% acetic acid, and the absorbance was measured at 405 nm.
Cellular senescence was determined using a β-galacto-sidase staining kit (Cell Biolabs, San Diego, CA, USA), following the manufacturer's instructions. Briefly, cells were washed with phosphate-buffered saline, fixed with 4% formaldehyde, and incubated overnight at 37℃ with β-galactosidase staining solution. Positive (blue) cells were observed under a light microscope. For quantitative evaluation of senescence, 15,000 cells were assayed using the Quantitative Cellular Senescence Assay Kit (Cell Biolabs, San Diego, CA, USA). Results were expressed as the relative fluorescence unit (RFU).
Intracellular ROS were measured using DCFDA Cellular ROS Detection Assay Kit (ab113852, Abcam, Cambridge, UK), following the manufacturer’s instructions. Cells (2×104) were seeded in each well of a 96-well plate and fluorescence was measured using a fluorescence plate reader (Biotek, Synergy H1; excitation=485 nm and emission=535 nm) to quantitate ROS production.
To determine oxidative DNA damage, 8-hydroxy-2’-deo-xyguanosine (8-OHdG) was measured using OxiSelect DNA Oxidative Damage ELISA Kit (STA-320, Cell Biolabs), following the manufacturer’s instructions. The absorbance was measured with a microplate reader at 450 nm.
Telomerase activity was determined using Telomerase PCR ELISA Kit (Roche Diagnostics Scandinavia AB, Stockholm, Sweden), following the manufacturer’s instruc-tions.
Total RNA was isolated using TRIzol (Invitrogen, Paisley, Scotland) according to the manufacturer’s instructions. The PCR reactions were performed on an Applied Biosystems StepOnePlusTM PCR machine using 5 μl SYBRⓇ Green PCR Master Mix (Life Technologies, Grand Island, NY, USA). Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used to quantify by specific marker genes. The primers used in this study are as follows. P15, p16, PCNA (proliferating cell nuclear antigen), PPARγ (peroxisome proliferator-activated receptor gamma), FABP4 (fatty acid-binding protein 4), ALPP (alkaline phosphatase), SPP1 (secreted phosphoprotein 1). All primers were obtained from Qiagen (Hilden, Germany). The delta-delta-Ct method was employed to determine the relative gene expression normalized to the endogenous controls glyceraldehyde-3-phosphate (GAPDH).
To determine whether culturing conditions affect aging-associated changes, we observed morphological changes in MSCs by culturing them at different cell densities for long term. Three different cell densities were selected: 50 (LD), 1,000 (MD), and 4,000 (HD) cells/cm2. Morphological changes in MSCs were observed from passage 5 to passage 15. At passage 15, HD showed flattened and enlarged shapes, which is the normally observed morphology of senescent cells. On the contrary, such changes were observed neither in LD nor in MD (Fig. 1a). To observe changes in cell size and granularity, we performed flow cytometry. At passage 5, no density-dependent difference in cell size was observed. However, at passages 10 and 15, cell size was significantly increased in HD compared to that in LD and MD. There was no difference in cell sizes between MD and LD (Fig. 1b). On the contrary, we found that granularity increased significantly with increasing cell density at all passages (Fig. 1c). These observations suggest that morphological changes in MSCs can be modulated by lowering cell density in long-term cultures.
To confirm whether the morphological changes shown in Fig. 1 were aging-dependent, cellular senescence was analyzed using senescence-associated β-galactosidase (SA-β-gal) staining. Cell density-dependent morphological changes in MSCs were not observed at passages 5 and 10. However, at passage 15, SA-β-gal positive cells were increased in HD compared to those in LD and MD (Fig. 2a). Quantitative analysis of fluorescence intensity in SA-β-gal positive cells showed the most prominent induction in HD at passage 15 (Fig. 2b).
Next, we performed experiments to observe differences in the expression of senescence-related genes, such as p15, p16, and proliferating cell nuclear antigen (PCNA), in MSCs cultured at three different cell densities. At passage 15, the expression of p15 and p16 was compared among LD, MD, and HD. We observed that the expression levels of both genes were decreased by lowering the cell densities. However, PCNA, a marker for proliferation, was decreased with increasing cell density (Fig. 2c). These results imply that senescence of MSCs can be delayed under low-density conditions.
As the proliferation ability of MSCs gradually decreases upon senescence, measuring proliferation ability is one of the tools for estimating senescence (22, 23). We analyzed proliferation potentials of cells at the abovementioned conditions of passage and cell density. FI, PDT, and PDL were calculated from initial cell number, final cell number, and culture period.
Throughout the passage stages, PDT tended to increase while both FI and PDL tended to decrease with increasing passage numbers at all densities. We noted that the decrease in proliferation ability during long-term culture was slower at low cell densities (Table 1). These results indicate that the lifespan of MSCs can be extended in vitro in long-term cultures with low density.
We analyzed differentiation potentials to check whether they were affected by varying cell densities. Both adipogenic and osteogenic differentiation potentials were compared among MSCs cultured at the abovementioned conditions. Although adipogenic potential, determined by Oil red O staining at passage 15 was decreased when compared to that at early passages, the difference was not significant across different cell densities (Fig. 3a and 3b). Adiopogenic differentiation potential of MSCs was also analyzed by specific marker expressions, such as PPARγ and FABP4. No significant difference across different cell densities was observed (Fig. 3c and 3d).
However, osteogenic differentiation potential, determined by Alizarin Red-S staining with mineralization quantitation was significantly decreased in a passage-dependent manner (Fig. 3e and 3f). There was no difference in differentiation potential regarding varying cell densities at passages 5 and 10. At passage 15, however, osteogenic differentiation potential was almost completely lost in HD but was maintained in LD and MD. It is well correlated with the reduction of osteogenic differentiation markers expressions, ALPP and SPP1, at passage 15, compared to those at passages 5 and 10. (Fig. 3g and 3h). These results indicate that osteogenic potential of MSCs can be maintained under low cell densities even after prolonged passage.
As cells become senescent, they show a direct correlation between decrease in stem cell function and accumulation of DNA damage (24-27). In particular, ROS induce oxidative DNA damage and decrease stem cell function, leading to MSC aging (25, 27). Accordingly, total cellular ROS were determined to identify whether ROS production was influenced by cell density. At passage 5, there were no significant differences in ROS production in LD, MD, and HD. On the contrary, at passages 10 and 15, ROS production was significantly decreased when cell density was lowered. Specifically, ROS production was significantly increased in HD compared to that in LD or MD (Fig. 4a). To confirm the above results, 8-OHdG, a DNA damage marker (28), was measured to assess ROS-dependent DNA damage. In our experiments, 8-OHdG production did not show any difference among the three cell densities at passages 5 and 10; however, at passages 15, it increased significantly with increase in cell density (Fig. 4b). These results indicate that DNA damage was induced by ROS at high cell density, resulting in increase in cell senescence.
To determine whether MSC proliferation was influenced by the ROS generated in high cell density cultures, HD were treated with AA, a well-known ROS scavenger. At passages 11∼15, AA was added to MSCs and their proliferative abilities were compared with those of untreated MSCs. At passage 11 of HD, FI values were 2.6±0.32 and 3.8±0.51 for AA-treated and untreated MSCs, respectively. At passage 15, the corresponding values were 1.6±0.14 and 2.5±0.27 (Fig. 4c). In addition, a decrease in ROS production due to AA treatment was confirmed at passage 15 (Fig. 4d). Overall, MSC proliferation ability was increased by ROS inhibition and it was maintained even after late passage. This suggests that the lifespan of MSCs can be improved via scavenging ROS. To confirm the above results, we examined the expressions of senescence-related genes, such as p15, p16, and PCNA in MSCs cultured at passage 15 of HD in the presence or absence of AA. According to scavenging ROS by treatment of AA, the expression levels of both p15 and p16 were decreased, but the expression of a proliferation marker, PCNA, was increased. (Fig. 4e). Moreover, the production of 8-OHdG, DNA damage marker, was significantly reduced by AA treatment, scavenging ROS (Fig. 4f). These results imply that senescence of MSCs can be delayed via scavenging ROS under high-density conditions.
MSCs have been under research focus because of their properties such as multi-lineage differentiation, self-renewal, and therapeutic effect on various diseases (29). However, MSCs have limited expansion potential in vitro; therefore, research aimed at developing a large-scale production technology is urgently needed (30-32). MSCs become senescent during repetitive passages and lose their proliferation potential and characteristics, leading to the loss of their unique properties, such as anti-inflammation response, immune modulatory function, and/or cell migration (14, 25). MSC aging is influenced by various parameters including ROS production, DNA damage, hindrance to protein homeostasis, and mitochondrial dysfunction (6, 19, 23). The lifespan of MSCs can be extended if senescence is controlled by manipulating these aging-associated phenomena.
In this study, we found that LD in long-term cultures show about seven times higher PDT and four times higher PDL at passage 15 than HD (Fig. 3a). These results indicate that the lifespan of MSCs in LD can be extended in vitro in long-term cultures and it can significantly increase their mass-culture productivity. Our results are consistent with those of previous studies, which reported that the proliferation activity and expression of stemness-related gene increase at low cell density (15, 17). Stemness of MSCs indicates not only proliferation ability but also the inherent functions, such as differentiation and colony formation. Gharibi et al. (4) reported that the inhibition of age-dependent phenomena improves not only proliferation ability but also adipogenic and osteogenic differentiation. However, our results showed that adipogenic differentiation potential was not influenced by cell density, whereas osteogenic differentiation potential was significantly decreased at passage 15 in HD compared to that in LD and MD (Fig. 3). This indicates that low cell density culture may also suppress the decrease of age-dependent differentiation potential in long-term culture of MSCs. Further research is needed to confirm whether low cell density regulates osteogenic differentiation.
ROS production and DNA damage can be key mechanisms underlying MSC senescence (19, 27). MSC aging is also expedited by decreasing telomerase activity (21, 26, 28). A decrease in ROS production and DNA damage has also been reported in previous studies in which MSC senescence was attenuated by treating the cells with ROS or autophagy inhibitors (12, 13). However, no attempt has been made to increase MSC proliferation activity by observing and controlling ROS production by modulating cell density. In this study, senescence of MSCs was delayed by decreasing ROS production and DNA damage in low cell density cultures. No significant difference was found in telomerase activity. The changes associated with low cell density are considered independent of telomerase activity. We confirmed that proliferation ability was restored by treating HD with AA (a ROS scavenger). We found that proliferation activity was further enhanced when ROS production was inhibited in the same manner as in LD (data not shown). This implies that senescence- dependent changes can be attenuated by inhibiting ROS produced during MSC expansion.
To verify whether the extended lifespan of MSCs cultured at low density increased the mass production yield and boosted the efficacy of MSCs in clinical settings, we need to produce MSCs by culturing at low density on a large-scale and test them in animal models. In addition, further research is needed to explore the molecular mechanism underlying cell density-dependent changes observed in MSCs at low cell density.
In conclusion, we demonstrated that delaying MSC senescence and extending their lifespan are possible by modulating cell density in vitro. We achieved these results without introducing any chemical additives/genetic vehicles or changing complicated culture methods. We believe that this method is an important piece of information for developing a large-scale production technology and enhancing MSC function.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
References
1. Both SK, van der Muijsenberg AJ, van Blitterswijk CA, de Boer J, de Bruijn JD. 2007; A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng. 13:3–9. DOI: 10.1089/ten.2005.0513. PMID: 17518576.

2. Koller MR, Emerson SG, Palsson BO. 1993; Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood. 82:378–384. DOI: 10.1182/blood.V82.2.378.378. PMID: 8329697.

3. Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. 2016; Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 23:76. DOI: 10.1186/s12929-016-0289-5. PMID: 27809910. PMCID: PMC5095977.

4. Gharibi B, Farzadi S, Ghuman M, Hughes FJ. 2014; Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells. 32:2256–2266. DOI: 10.1002/stem.1709. PMID: 24659476.

5. de Witte SFH, Lambert EE, Merino A, Strini T, Douben HJCW, O'Flynn L, Elliman SJ, de Klein AJEMM, Newsome PN, Baan CC, Hoogduijn MJ. 2017; Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy. 19:798–807. DOI: 10.1016/j.jcyt.2017.03.071. PMID: 28462821.

6. Sethe S, Scutt A, Stolzing A. 2006; Aging of mesenchymal stem cells. Ageing Res Rev. 5:91–116. DOI: 10.1016/j.arr.2005.10.001. PMID: 16310414.

7. Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N, Dalloul A. 2011; Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 12:12. DOI: 10.1186/1471-2121-12-12. PMID: 21450070. PMCID: PMC3073900.

8. Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P, Molaeipour Z, Saleh M. 2015; The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 5:141–149. DOI: 10.15171/apb.2015.021. PMID: 26236651. PMCID: PMC4517092.

9. Khan M, Akhtar S, Mohsin S, N Khan S, Riazuddin S. 2011; Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 20:67–75. DOI: 10.1089/scd.2009.0397. PMID: 20446810.

10. Kim JH, Kim WK, Sung YK, Kwack MH, Song SY, Choi JS, Park SG, Yi T, Lee HJ, Kim DD, Seo HM, Song SU, Sung JH. 2014; The molecular mechanism underlying the proliferating and preconditioning effect of vitamin C on adipose-derived stem cells. Stem Cells Dev. 23:1364–1376. DOI: 10.1089/scd.2013.0460. PMID: 24524758. PMCID: PMC4046194.

11. Rodrigues M, Griffith LG, Wells A. 2010; Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther. 1:32. DOI: 10.1186/scrt32. PMID: 20977782. PMCID: PMC2983445.

12. Misra J, Mohanty ST, Madan S, Fernandes JA, Hal Ebetino F, Russell RG, Bellantuono I. 2016; Zoledronate attenuates accumulation of DNA damage in mesenchymal stem cells and protects their function. Stem Cells. 34:756–767. DOI: 10.1002/stem.2255. PMID: 26679354. PMCID: PMC4832316.

13. Oh J, Lee YD, Wagers AJ. 2014; Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 20:870–880. DOI: 10.1038/nm.3651. PMID: 25100532. PMCID: PMC4160113.

14. Schultz MB, Sinclair DA. 2016; When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 143:3–14. DOI: 10.1242/dev.130633. PMID: 26732838. PMCID: PMC4725211.

15. Kim DS, Lee MW, Ko YJ, Chun YH, Kim HJ, Sung KW, Koo HH, Yoo KH. 2016; Cell culture density affects the proliferation activity of human adipose tissue stem cells. Cell Biochem Funct. 34:16–24. DOI: 10.1002/cbf.3158. PMID: 26778408.

16. Kim DS, Lee MW, Yoo KH, Lee TH, Kim HJ, Jang IK, Chun YH, Kim HJ, Park SJ, Lee SH, Son MH, Jung HL, Sung KW, Koo HH. 2014; Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 9:e83363. DOI: 10.1371/journal.pone.0083363. PMID: 24400072. PMCID: PMC3882209.

17. Kim DS, Lee MW, Lee TH, Sung KW, Koo HH, Yoo KH. 2017; Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed Rep. 6:300–306. DOI: 10.3892/br.2017.845. PMID: 28451390. PMCID: PMC5403436.

18. Jeon MS, Yi TG, Lim HJ, Moon SH, Lee MH, Kang JS, Kim CS, Lee DH, Song SU. 2011; Characterization of mouse clonal mesenchymal stem cell lines established by subfrac-tionation culturing method. World J Stem Cells. 3:70–82. DOI: 10.4252/wjsc.v3.i8.70. PMID: 22007272. PMCID: PMC3192225.

19. Signer RA, Morrison SJ. 2013; Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 12:152–165. DOI: 10.1016/j.stem.2013.01.001. PMID: 23395443. PMCID: PMC3641677.

20. Zhao Q, Wang XY, Yu XX, Zhai YX, He X, Wu S, Shi YA. 2015; Expression of human telomerase reverse transcriptase mediates the senescence of mesenchymal stem cells through the PI3K/AKT signaling pathway. Int J Mol Med. 36:857–864. DOI: 10.3892/ijmm.2015.2284. PMID: 26178664.

21. Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. 2003; Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 17:1146–1149. DOI: 10.1038/sj.leu.2402962. PMID: 12764382.

22. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. 2008; Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 3:e2213. DOI: 10.1371/journal.pone.0002213. PMID: 18493317. PMCID: PMC2374903.

23. Feng G, Tan W, Gu Z. 2013; Mesenchymal stem cells and senescence. Clon Transgen. 2:104. DOI: 10.4172/2168-9849.1000104.

24. Jacobs K, Zambelli F, Mertzanidou A, Smolders I, Geens M, Nguyen HT, Barbé L, Sermon K, Spits C. 2016; Higher-density culture in human embryonic stem cells results in DNA damage and genome instability. Stem Cell Reports. 6:330–341. DOI: 10.1016/j.stemcr.2016.01.015. PMID: 26923824. PMCID: PMC4788786.

25. Yang SR, Park JR, Kang KS. 2015; Reactive oxygen species in mesenchymal stem cell aging: implication to lung diseases. Oxid Med Cell Longev. 2015:486263. DOI: 10.1155/2015/486263. PMID: 26273422. PMCID: PMC4529978.

26. Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. 2017; Senescence of mesenchymal stem cells (Review). Int J Mol Med. 39:775–782. DOI: 10.3892/ijmm.2017.2912. PMID: 28290609.

27. Denu RA, Hematti P. 2016; Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. 2016:2989076. DOI: 10.1155/2016/2989076. PMID: 27413419. PMCID: PMC4928004.

28. Vono R, Jover Garcia E, Spinetti G, Madeddu P. 2018; Oxidative stress in mesenchymal stem cell senescence: regulation by coding and noncoding RNAs. Antioxid Redox Signal. 29:864–879. DOI: 10.1089/ars.2017.7294. PMID: 28762752. PMCID: PMC6080119.

29. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. 2013; Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 34:747–754. DOI: 10.1038/aps.2013.50. PMID: 23736003. PMCID: PMC4002895.

30. Bartmann C, Rohde E, Schallmoser K, Pürstner P, Lanzer G, Linkesch W, Strunk D. 2007; Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 47:1426–1435. DOI: 10.1111/j.1537-2995.2007.01219.x. PMID: 17655587.

31. Colter DC, Class R, DiGirolamo CM, Prockop DJ. 2000; Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 97:3213–3218. DOI: 10.1073/pnas.97.7.3213. PMID: 10725391. PMCID: PMC16218.

32. Fekete N, Rojewski MT, Fürst D, Kreja L, Ignatius A, Dausend J, Schrezenmeier H. 2012; GMP-compliant isolation and large-scale expansion of bone marrow-derived MSC. PLoS One. 7:e43255. DOI: 10.1371/journal.pone.0043255. PMID: 22905242. PMCID: PMC3419200.

Fig. 1
Cell culturing density-dependent morphological changes in MSCs in long-term cultures. (a) At passages 5, 10, and 15, morphological changes were observed in MSCs under three different cell densities. Scale bar: 200 μm. LD=50 cells/cm2, MD=1000 cells/cm2, and HD=4000 cells/cm2. (b, c) Cell size and granularity were analyzed using flow cytometry. FSC=forward scatter and SSC=side scatter. Significance is *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
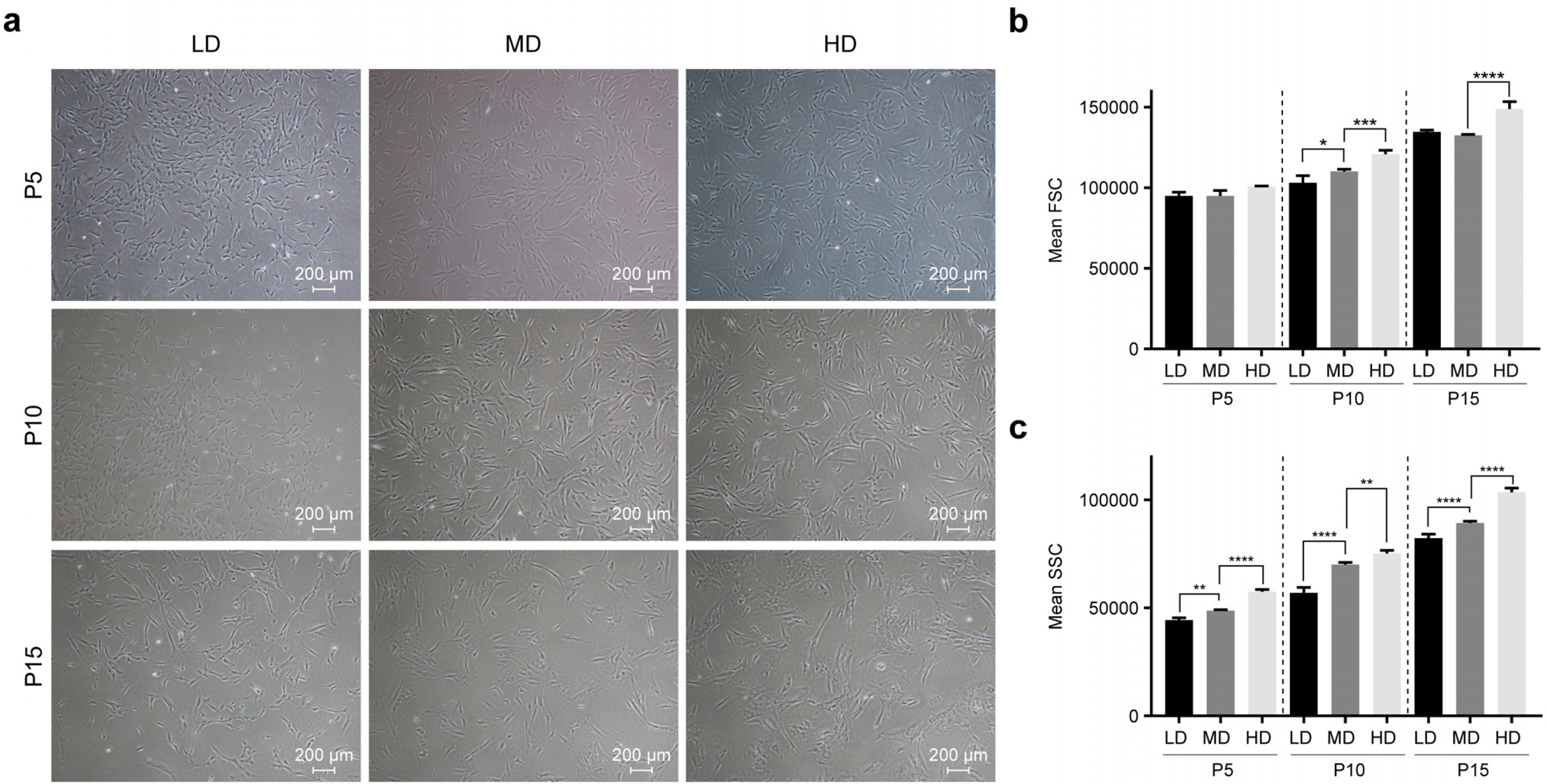
Fig. 2
Cell density-dependent changes in senescence of MSCs in long-term cultures. (a) At passages 5, 10, and 15, senescence changes were observed in LD, MD, and HD using β-galactosidase staining. Scale bar: 500 μm. (b) Quantification of SA-β-gal activity in LD, MD, and HD. Senescence change was measured using the quantitative cellular senescence assay kit and expressed as relative fluorescence unit (RFU). (c) At passage 15, transcriptional changes in p15 and p16 genes, which are associated with senescence, and proliferating cell nuclear antigen (PCNA), a marker for cell proliferation, were analyzed using quantitative real-time PCR in LD, MD, and HD. (d) Changes in telomerase activities were measured using a Telomerase PCR ELISA Kit in LD, MD, and HD. Significance is *p<0.05, **p<0.01, ****p<0.0001.
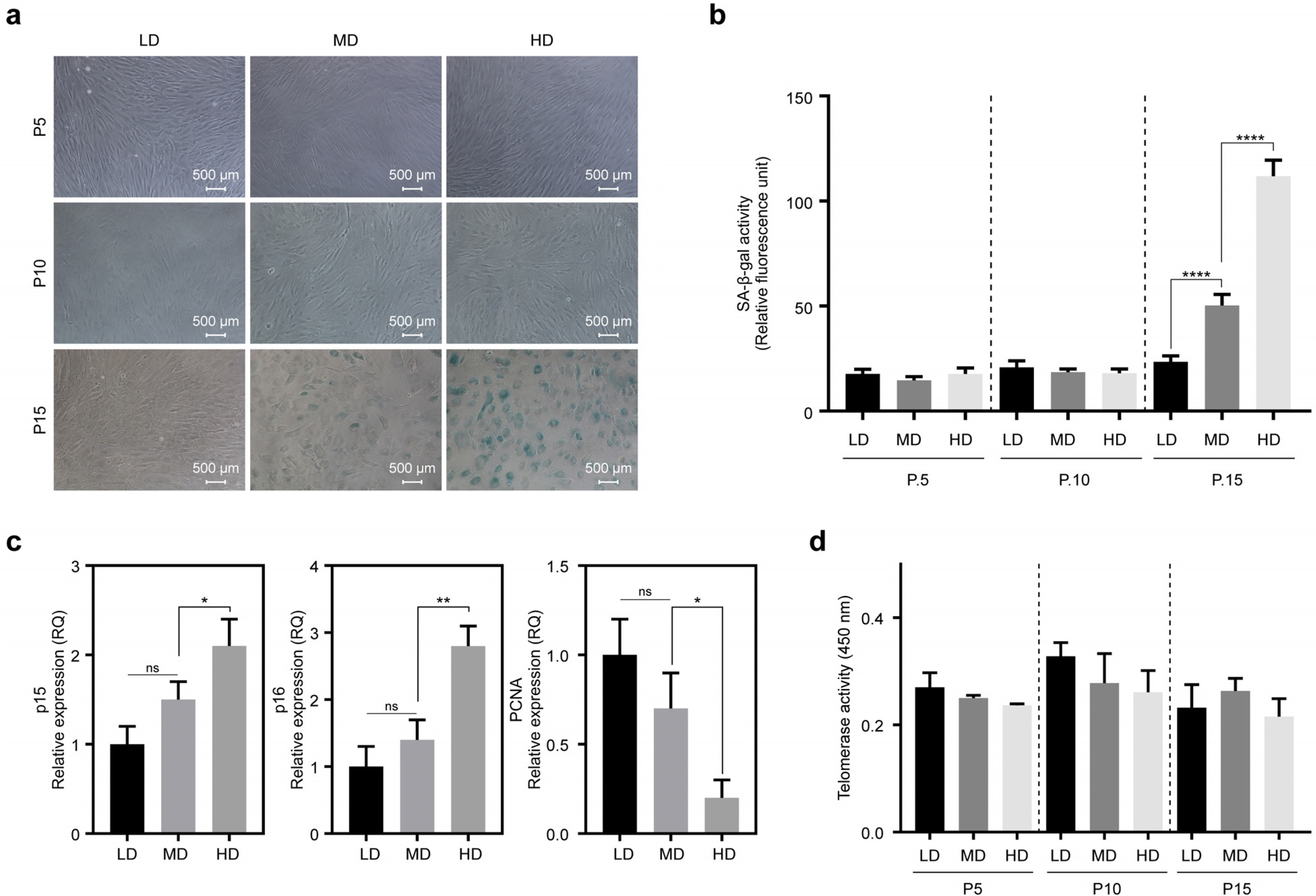
Fig. 3
Cell density-dependent changes in the lifespan of MSCs in long-term cultures. (a, b) Adipogenic differentiation potentials were compared among LD, MD, and HD. The cells were visualized by staining with Oil red O. After de-staining with 100% isopropanol, samples were quantified by measuring absorbance at 500 nm. (c, d) Quantitative PCR analysis of adipogenic differentiation markers (PPARγ, FABP4) in LD, MD, and HD. Graphs are represented as relative expression units compared with GAPDH. (e, f) Osteogenic differentiation potentials were compared among LD, MD, and HD. The cells were visualized by staining with Alizarin red S. After de-staining with 10% acetic acid, samples were quantified by measuring absorbance at 405 nm. (g, h) Quantitative PCR analysis of osteogenic differentiation markers (ALPP, SPP1) in LD, MD, and HD. Graphs are represented as relative expression units compared with GAPDH. Significance is *p<0.05, **p<0.01.
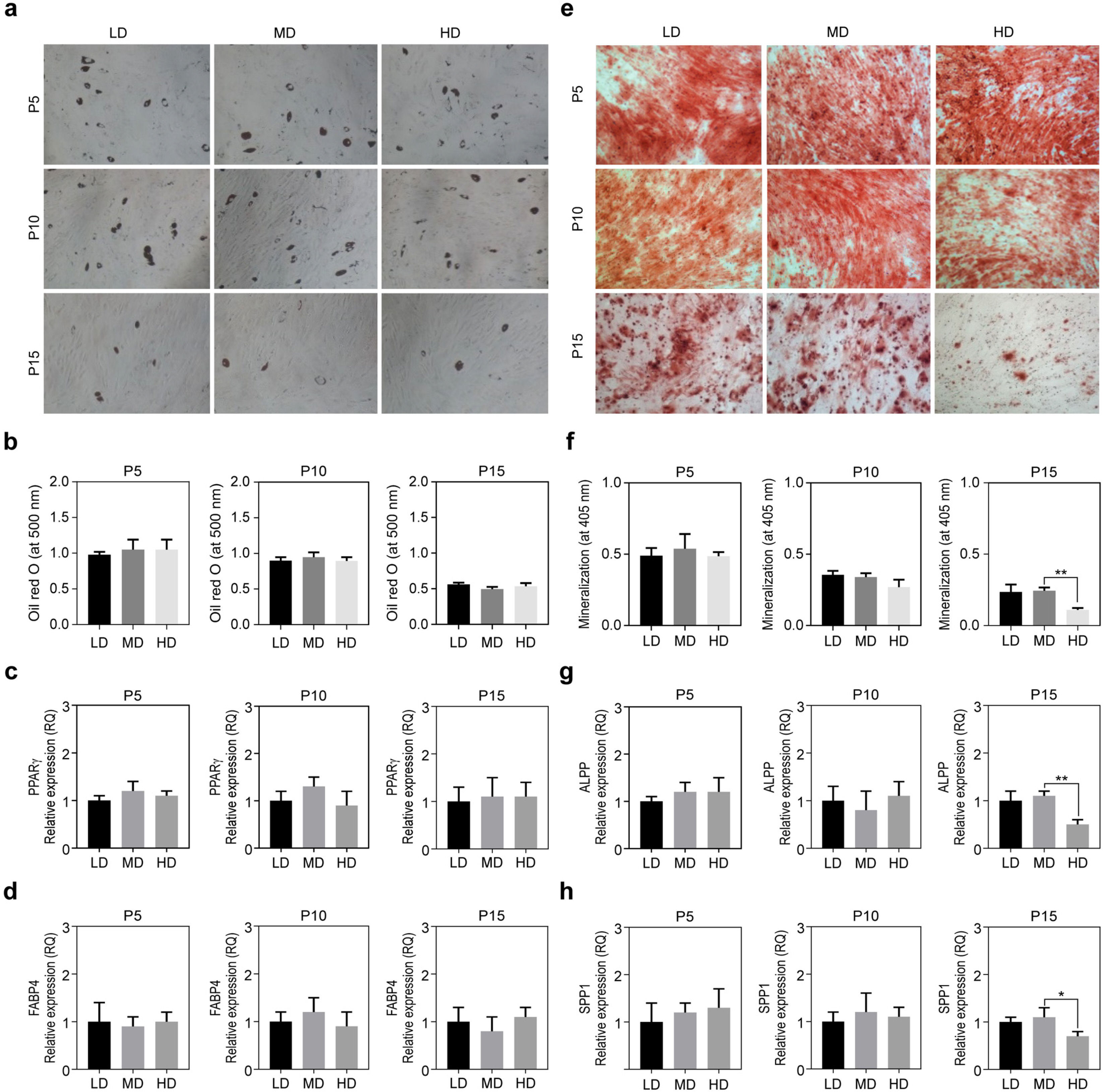
Fig. 4
Cell density-dependent production of total ROS and DNA damage in long-term cultures. (a) Total ROS were detected using 2’, 7’-dichlorofluorescein diacetate (DCFDA) assay. MSCs were treated with 10 μM DCFDA solution and fluorescence was quantified using a fluorescence reader (excitation=485 nm and emission=535 nm). (b) Oxidative DNA damage was analyzed by measuring 8-OHdG produced in LD, MD, and HD. (c) MSC proliferation was compared among HD at passages 11, 13, and 15, with and without AA (Ascorbic acid, 25 μg/ml) treatment. FI and relative proliferation were calculated by cell counting. (d) At passage 15, ROS generation was analyzed in HD treated with or without AA by staining with DCFDA. (e) At passage 15, p15, p16, and PCNA expressions were analyzed in HD treated with or without AA by Quantitative PCR. (f) At passage 15, Oxidative DNA damage was analyzed in HD treated with or without AA by measuring 8-OHdG production. Significance is *p<0.05, **p<0.01, ****p<0.0001.

Table 1
Effect of different cell densities on the lifespan of MSCs in long-term cultures




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download