Introduction
From feeder to feeder-free system for hPSCs culture
Matrices for feeder-free system
Test of chemically defined system for hPSCs culture
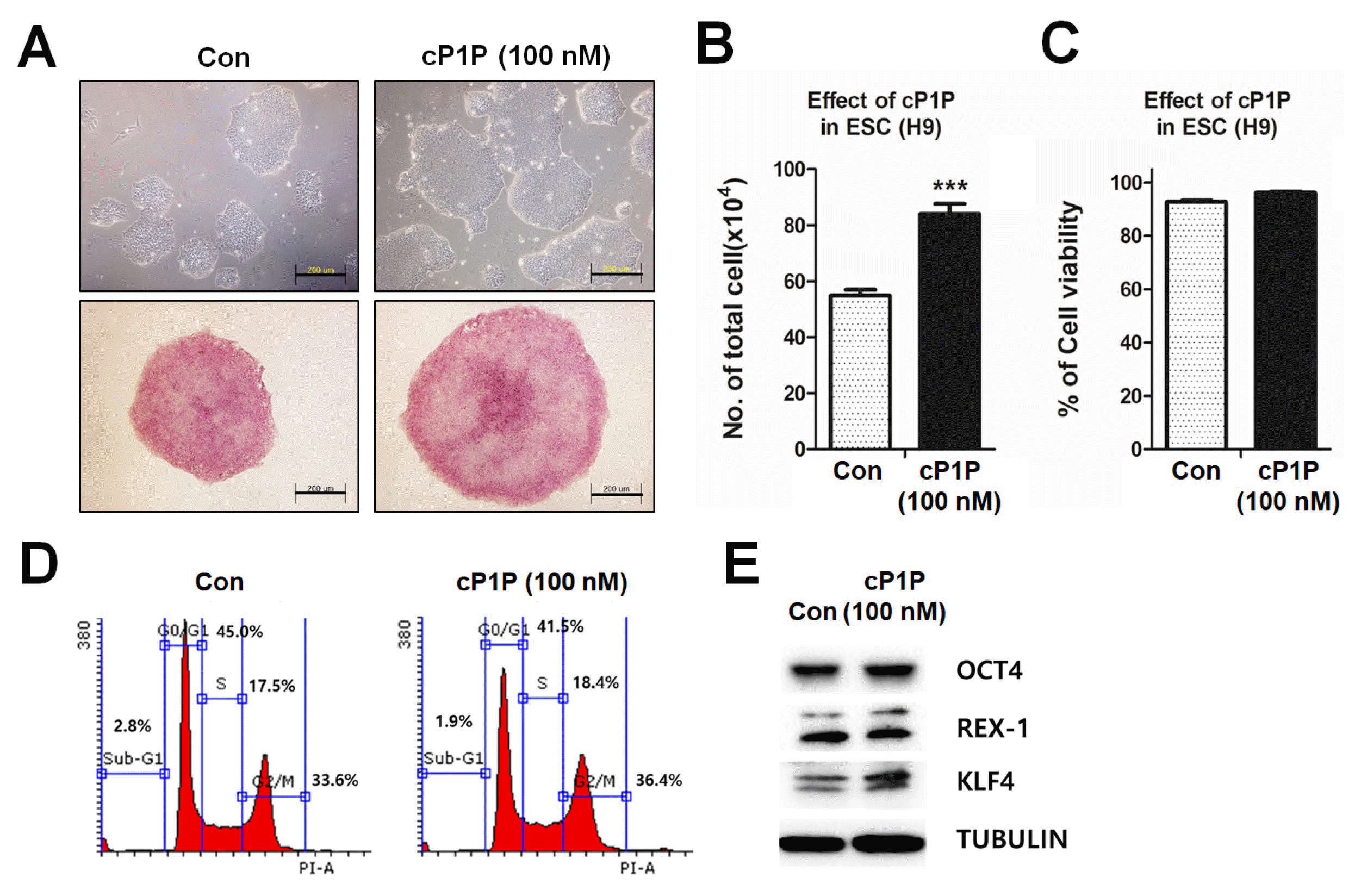
Supplement of bioactive lysophospholipid for improving proliferation of hPSCs
Materials and Methods
Cell lines
Reagents
ROCK Inhibitor Y-27632 (Tocris, 10 mg, cat. no. 1254, UK)
Vitronectin XF™ (STEMCELL Technologies, 2 ml, cat. no. 07180, Canada)
CellAdhere™ Dilution Buffer (STEMCELL Technologies, cat. no. 07183, Canada)
TeSR™-E8™ Kit for hESC/hiPSC Maintenance (STEMCELL Technologies, 05940, Canada) contains TeSR™-E8™ Basal Medium (cat. no. 05991) and TeSR™ -E8™ 25X Supplement (cat. no. 05992)
DPBS (PAN BIOTECH, cat. no. P04-36500, Germany)
Cryostor CS10 (STEMCELL Technologies, cat. no. 07930, Canada)
TrypLE™ Select Enzyme (Life Technologies, cat. no. 12605-010, US)
Gentle Cell Dissociation Reagent (STEMCELL Technologies, cat. no. 07174, Canada)
STEMdiff™ Trilineage Differentiation kit (STEMCELL Technologies, 05230, Canada)
O-cyclic Phytosphingosine-1-phosphate (cP1P, AXCESO BIOPHAMA, Korea)
70% Ethanol (MERCK, cat. no. 1.00974.1011, Germany)
Isopropanol (MERCK, cat. no. 1.09634.2511, Germany)
Equipment
Safety bench, Class II (ILJIN HI-TECH, Korea)
Refrigerator
Freezer, −20/−80°C (Panasonic, MDF-u54v-pk, Japan)
Micropipettes (GILSON, USA)
Sterile pipette tips, 100, 200 and 1,000 μl (SARSTEDT,
Germany)
CO2 incubator (Thermo Scientific, HERA CELL 240i, USA)
Water bath, 37°C (SAMHEUNG ENERGY, WB-11GDN, Korea)
Centrifuge (Eppendorf, 5810R, Germany)
Hood equipped with stereomicroscope (OLYMPUS, SZ61, Japan)
Light microscope (OLYMPUS, CKX41, Japan)
Liquid nitrogen storage tank (CRYO Industries, USA)
Conical tube, 15 ml (SPL, cat. no. 50015, Korea)
Conical sterile polypropylene tube, 50 ml (SPL, cat. no. 50050, Korea)
Sterile serological pipettes, 5, 10 and 50 ml (SPL, cat. no. 91005, 91010 and 91050, Korea)
35×10 mm dish (BD FALCON, cat. no. 353001, USA)
6-well plate (CORNING, cat. no. CT-3516, USA)
Cryogenic handling gloves (Honeywell, USA)
Forceps (WORLD PRECISION INSTRUMENTS, USA)
Isopropanol freezing containers (Thermo Scientific, cat. no. 5100-0001, USA)
Cryovials, 2.0 ml (CORNING, cat. no. CC-430488, USA)
Cryo 1°C freezing container (Nalgene, cat. no. 52100-0001)
Equipment setup
Complete TeSR™-E8™ medium
cP1P
Vitronectin XFTM
▶ Note: A 10 μg/ml of working concentration is sufficient to support most of hPSC lines. However, the optimal concentration may vary depending on the cell line.
▶ Note: We make 240 μl aliquots of the stock solution in 1.5 ml sterile effendorf tube for coating one 6-well plate (1 ml/well). The aliquots and coating volume may vary depending on the scale of the culture and cultureware. Once diluted, use immediately within 1 week and do not re-freeze.
ROCK inhibitor
▶ Note: If MW of material is not 320.26, dilute appropriately to achieve 10 mM solution. Batch specific MWs may vary from batch to batch due to the degree of hydration, which will affect the solvent volumes required to prepare stock solution.
▶ Note: Normally we do not recommend the use of ROCK inhibitor. This could be an option for users to enhance cell survival after single cell dissociation for passaging or during initial thawing of cryopreserved hPSCs, as it has been reported. Stock solution should be diluted into culture medium immediately before use.
Procedure
Matrix and medium preparation (outlining how to coat six wells of non-tissue culture-treated 6-well plate)
1) Thaw an aliquot (240 μl) of Vitronectin XFTM stock solution at room temperature.
▶ Note: Avoid additional freeze-thaw cycle.
2) Dilute 240 μl of Vitronectin XFTM stock solution in 5.76 ml of CellAdhereTM Dilution Buffer or DPBS to make 6 ml of a working solution (10 μg/ml) and mix gently (Do not vortex).
3) Add 1 ml of diluted working solution to the center of each well of 6-well plate and gently rock the plate to evenly distribute the solution across the surface.
4) Incubate the plate at room temperature for a minimum 2 h prior to use.
▶ Note: If not used immediately, the plates can be prepared for later use. The plates must be sealed tightly with Parafilm® to prevent evaporation and stored at 2~ 8°C for up to 1 week. Allow stored coated plates to come to room temperature for 30 min prior to proceeding.
5) While incubation of the coated plates, warm complete TeSRTM-E8TM medium at room temperature (15~ 25°C). Do not warm medium in a 37°C water bath.
▶ Note: The complete TeSRTM-E8TM medium is light and temperature sensitive. Do not leave medium at room temperature for longer than 2 h and avoid exposure to light to prevent degradation of medium components.
6) To prepare the plates for passaging or thawing of hPSCs, remove the excess vitronectin from the plates and wash with CellAdhereTM dilution buffer or DPBS.
7) Aspirate wash solution and add 2 ml of pre-warmed TeSRTM-E8TM medium per well.
8) Place the plates in a 37°C, 5% CO2 incubator 1 h before thawing or passaging.
Thawing hPSCs
1) Take the cryovial out of liquid nitrogen and move them to the tissue culture room on ice.
2) Quickly thaw the cells by gently swirling the vial in a 37°C water bath.
3) When only a small particle of ice is left in the vial, wipe the outside of the cryovial with 70% (v/v) ethanol to disinfect, dry and bring it under a biosafety cabinet.
4) Gently transfer the aggregates suspension into a 15 ml conical tube using a 1 or 2 ml serological pipette to minimize breakage of cell aggregates.
5) Add 5~7 ml of pre-warmed TeSRTM-E8TM medium in a drop-wise manner to the tube.
▶ Note: While adding drops, gently rock the tube back and forth to minimize osmotic shock to the cells.
6) Centrifuge the mixture at 150 g for 3 min at room temperature.
7) Discard the supernatant and re-suspend the aggregates in 1 ml of pre-warmed TeSRTM-E8TM medium.
▶ Note: While slowly adding warm TeSRTM-E8TM medium, gently tap the tube to dislodge the cell pellet.
8) Plate the aggregates into one to two wells of vitronectin-coated plate and gently move the plate side to side, back and forth to distribute the aggregates evenly.
▶ Note: Supplement the medium with a stock solution of ROCK inhibitor to maintain 10 μM final concentration (add 1 μl of 10 mM ROCK inhibitor for 1 ml of medium) for enhancing cell survival after plating.
9) Incubate the plate at 37°C, 5% CO2 incubator and do not disturb the plates for 24 h after plating.
Passaging of hPSCs
1. Passaging as cell aggregates
1) Remove spent medium from the culture vessel and rinse with DPBS.
2) Add 1 ml of Gentle Cell Dissociation Reagent (GCDR) solution and incubate for 2~6 min at room temperature (15~25°C).
▶ Note: Observe cells under microscope during incubation to remove the GCDR solution before the colonies are completely detached. The incubation times may vary depending on cell lines.
3) Aspirate the GCDR solution and rinse with 0.5 ml of pre-warmed TeSRTM-E8TM medium.
4) Add fresh 1 ml of warm TeSRTM-E8TM medium.
5) Gently scrape the colonies with a 5 ml serological pipette or cell scraper and transfer the detached aggregates to a 15 ml conical tube using a 2 ml serological pipette.
6) Triturate the aggregates to create appropriate size (approximately 50~200 μm) for plating with a 200 μl or 1 ml pipette tips.
7) Plate the small aggregates at the desired density onto vitronectin-coated plates containing the complete TeSRTME8TM medium.
▶ Note: The split ratio should be determined depending on the confluency at the day of passaging and the growth rates of cell lines.
8) Gently move the plate side to side, back and forth to distribute the aggregates evenly.
9) Incubate the plate at 37°C, 5% CO2 incubator and do not disturb the plates for 24 h after plating.
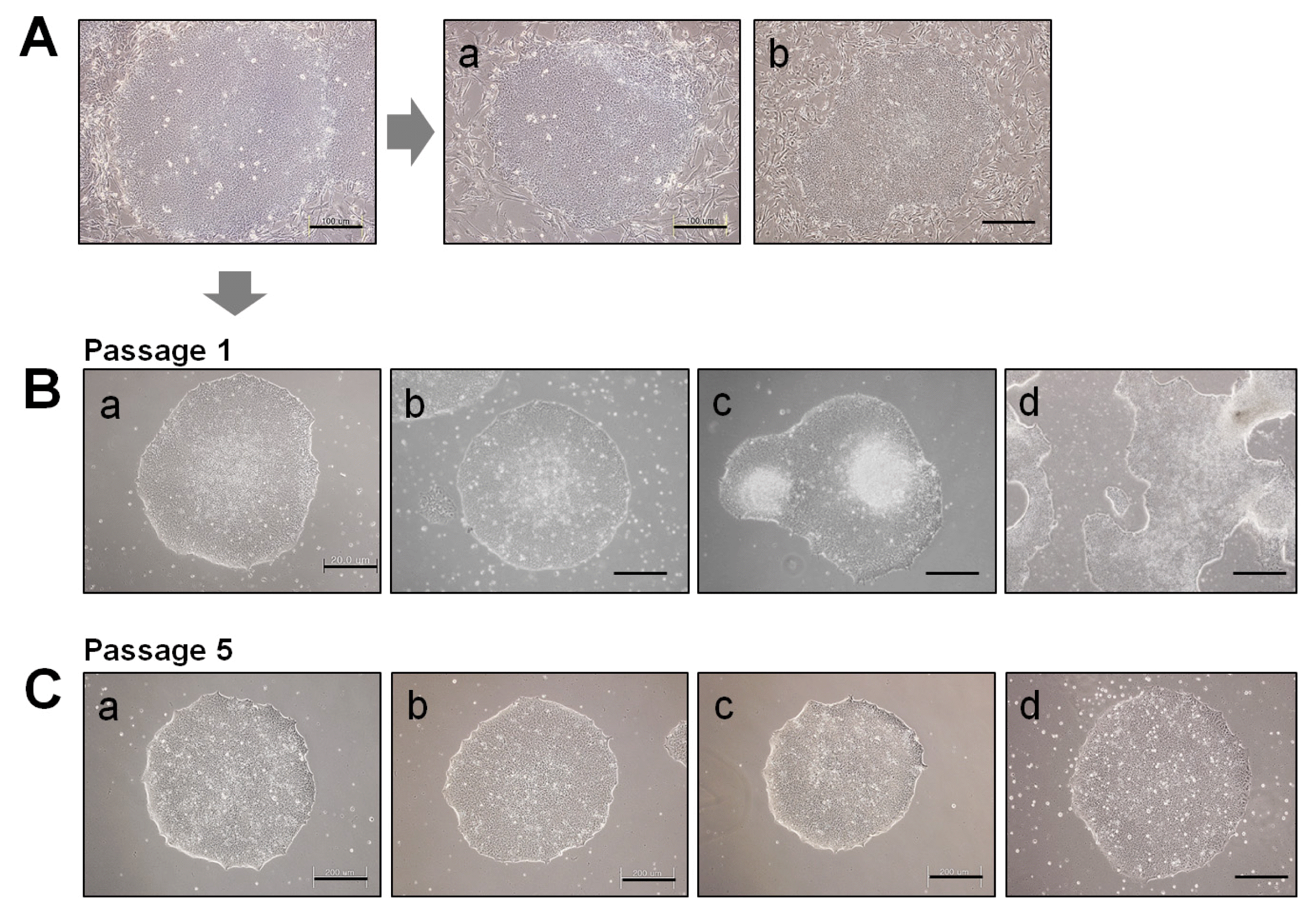
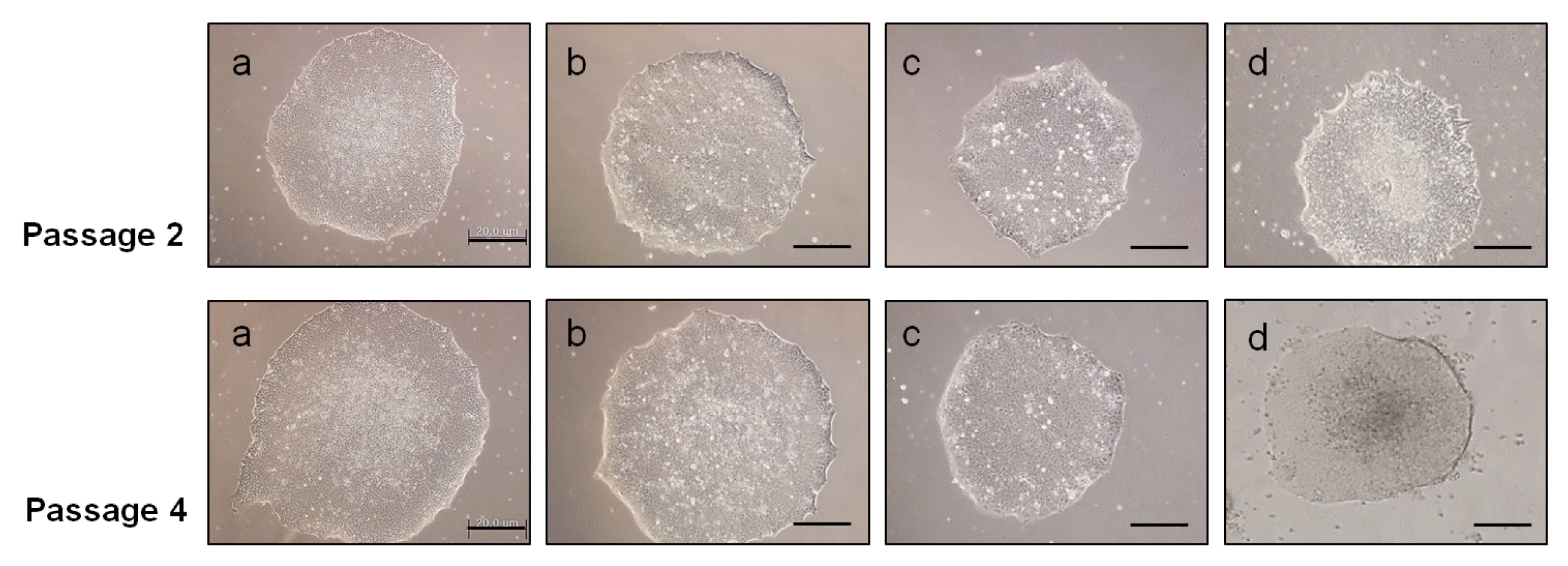
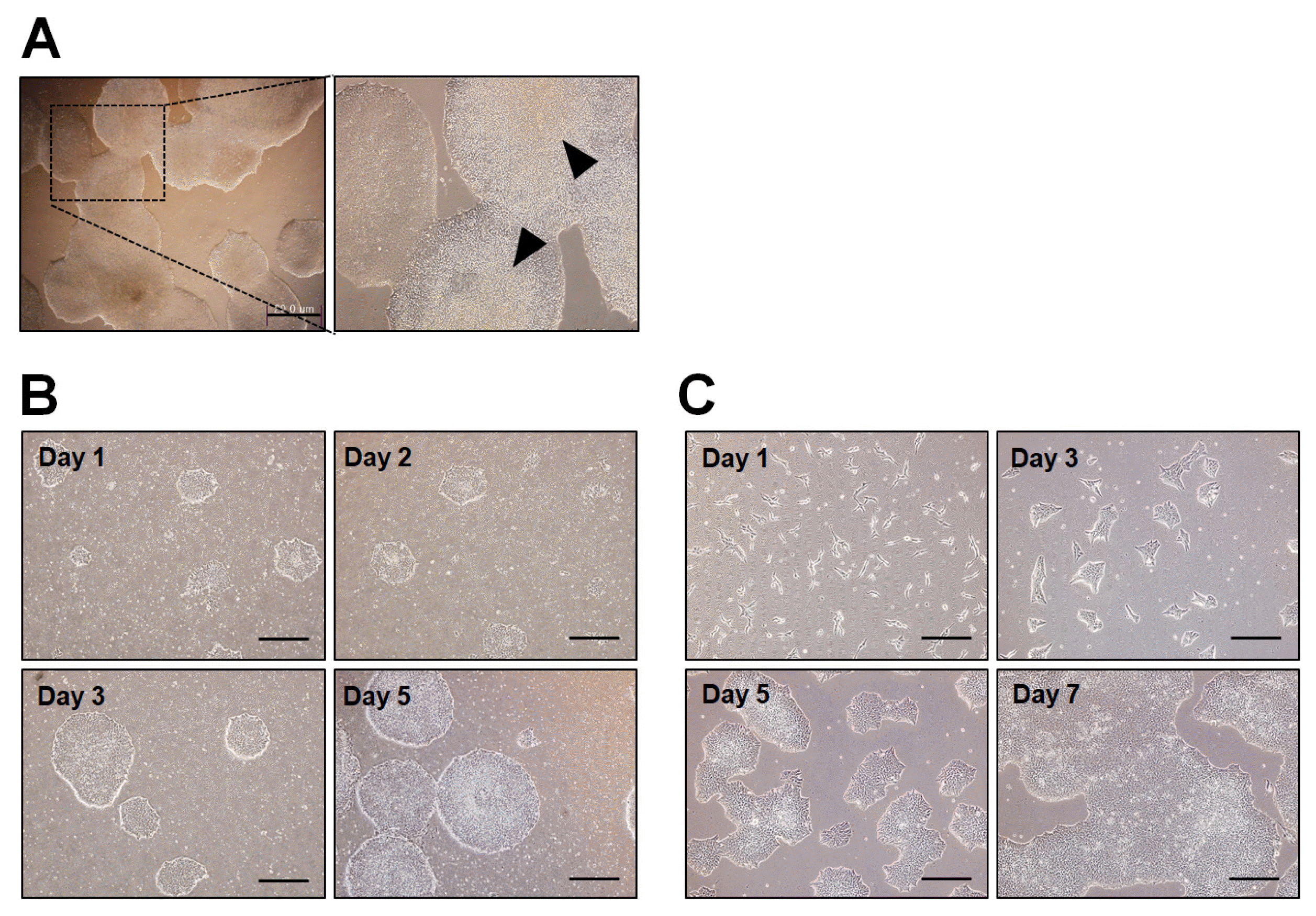
10) Feed daily with fresh medium and observe regularly cell growth and morphology (Fig. 4B).
2. Passaging as single cell suspensions
1) Remove spent medium from the culture vessel and rinse with DPBS.
2) Add 1 ml of pre-warmed TrypLE to wells and incubate for 5 min at 37°C, 5% CO2 incubator.
3) Aspirate the TrypLE solution and rinse with 0.5 ml of pre-warmed TeSRTM-E8TM medium.
▶ Note: The rinsing should be done carefully as the colonies are loosely attached after the TrypLE treatment for 5 min.
4) Add fresh 1 ml of warm TeSRTM-E8TM medium and break the colonies to single cell suspensions by gentle trituration with a 1 ml pipette tip.
5) Count the cells using a hemocytometer. Plate the cells at densities of 30,000~50,000 cells per cm2 onto vitronectin-coated plates containing the complete TeSRTM-E8TM medium.
▶ Note: Addition of 10 mM ROCK inhibitor (1 μl of 10 mM ROCK inhibitor per 1 ml of medium) is recommended when plating single cell suspensions for enhancing cell survival.
▶ Note: Seeding density is critical for the outcome of the passaging. Thus, optimal seeding density should be adjusted empirically for each culture condition (matrix, medium, and culture ware) and each cell line.
6) Gently move the plate side to side, back and forth to distribute the single cell suspensions evenly.
7) Incubate the plate at 37°C, 5% CO2 incubator and do not disturb the plates for 24 h after plating.
8) Feed daily with fresh medium and observe regularly cell growth and morphology (Fig. 4C).
Freezing of hPSCs (outlining how to freeze hPSCs that maintained under above culture conditions in cell aggregates)
1) Remove spent medium from the culture vessel and rinse with DPBS.
2) Add 1 ml of GCDR and incubate for 6~12 min at 37°C, 5% CO2 incubator.
▶ Note: Observe cells under microscope during incubation to remove the GCDR before the colonies are completely detached. The incubation times may vary depending on cell lines.
3) Aspirate the GCDR and add fresh warm TeSRTME8TM medium.
4) Gently scrape the colonies with a 5 ml serological pipette or cell scraper.
▶ Note: Do not use a pipette to avoid breaking the cell aggregates into small pieces or single cells. Leave the aggregates as large as possible.
5) Gently transfer the aggregates suspension into a 15 ml conical tube using a 1 or 2 ml serological pipette to minimize breakage of cell aggregates.
6) Centrifuge at 150 g for 3 min at room temperature.
7) Gently aspirate the supernatant and re-suspend the aggregates in 1 ml of cold CryoStor® CS10.
▶ Note: While slowly adding cryopropectant, gently tap the tube to dislodge the cell pellet. Care must be taken to minimize the breakup of the aggregates.
8) Transfer the 1 ml suspension of aggregates in a cryovial using a 1 or 2 ml serological pipette.
9) Immediately place the cryovials into a pre-chilled freezing container and keep the aggregates at −20°C for 2 h, followed by −80°C for 2 h.
10) Transfer the aggregates into a liquid nitrogen tank for long-term storage.
▶ Note: A standard slow rate-controlled cooling protocol can be used for freezing (i.e. Reduce temperature at approximately −1°C/min, followed by long-term storage in liquid nitrogen).
Routine characterization for long-term maintenance of hPSCs
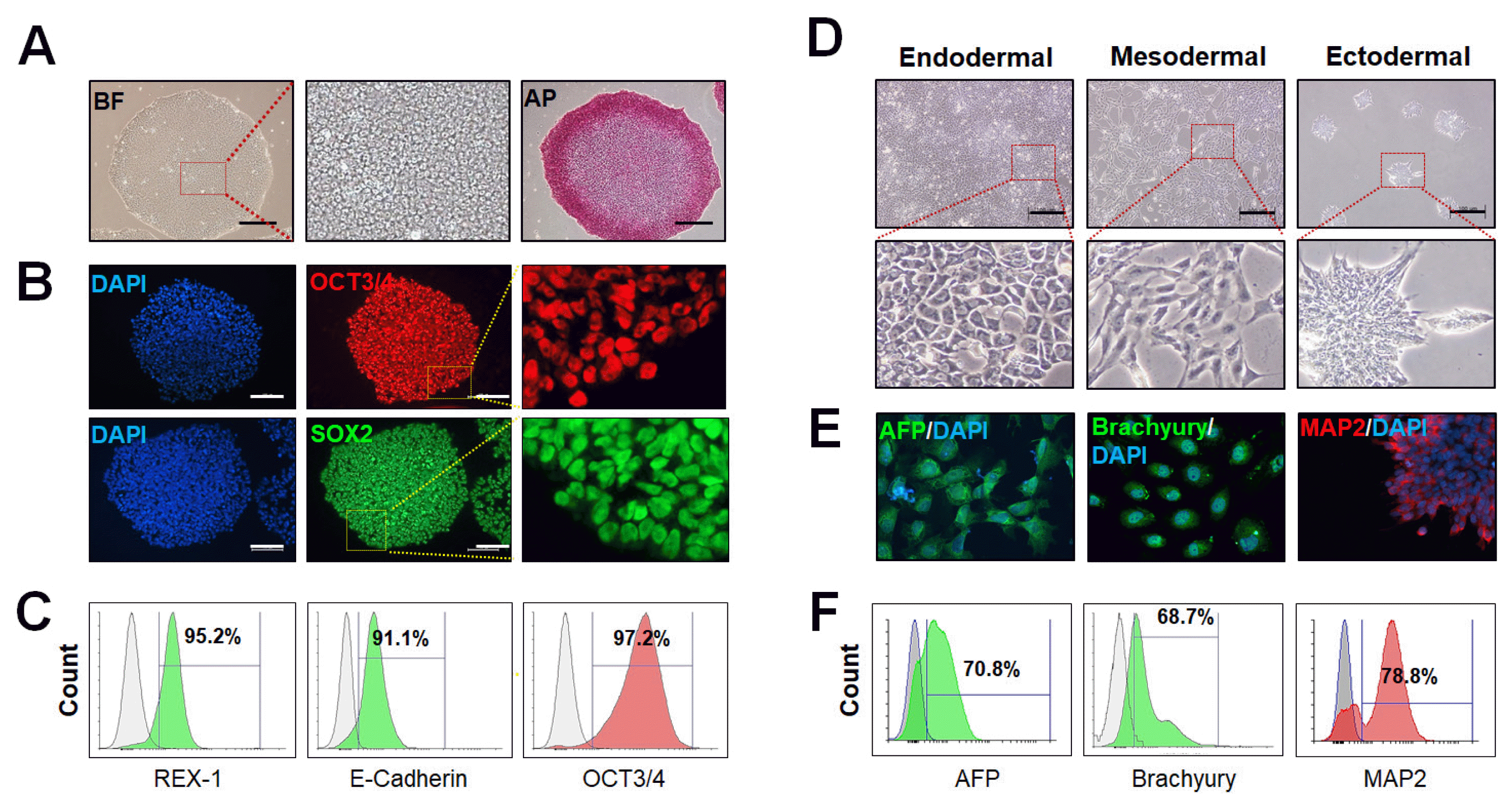
1. Pluripotency marker expression by immunofluorescence staining (Fig. 5A, 5B)
1) Aspirate the spent medium.
2) Fix the cells with 4% PFA in PBS pH 7.4 for 10 min at room temperature.
3) Incubate the samples for 10~30 min with PBS containing 0.1~0.4% Triton X-100. Optimal percentage of Triton X-100 should be determined for each protein of interest.
4) Incubate cells with 1% BSA in PBS for 1 h to block unspecific binding of the antibodies. Alternative blocking solution may use 1~10% serum from a goat or donkey; see antibody datasheet for recommendations.
5) Incubate cells in the diluted primary antibody with blocking solution in a humidified chamber for 1 h at room temperature or overnight at 4°C.
6) Discard the solution and wash the cells three times in PBS, 5 min each wash.
7) Incubate cells with the secondary antibody in PBS for 1 h at room temperature in the dark.
8) Repeat step 6.
9) Counter staining with 0.1~1 μg/ml Hoechst or DAPI for 5 min in the dark.
10) Rinse with PBS.
11) Analyze samples.
2. Pluripotency marker expression by flow cytometry (Fig. 5C)
1) Harvest, wash the dissociate cells (1~5×106 cells/ml) that were fixed in 100 μl of 1% PFA with 0.1~0.4% Triton X-100 or Tween 20 in cold PBS to each sample, then mix gently and incubate for 10~15 min.
2) Cell down (centrifuge 400~600 ×g for 5 min at room temperature) and discard the supernatant.
3) Incubate cells with 1% BSA in cold PBS for 1 h to block unspecific binding of antibodies on ice.
4) Repeat step 2.
5) Re-suspend the cell pellet in 100 μl of 1% BSA-PBS with directly conjugated primary antibody and incubate for 30~60 min at room temperature. Protect form light.
6) Repeat step 2.
7) Re-suspend stained cells in an appropriate volume of PBS or FACS buffer.
8) Analyze samples.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download