Introduction
Materials and Methods
PVC isolation and cultures
Preparation of PVC-conditioned medium
Cell culture and inflammasome activation
Western blot analysis
Cytotoxicity test
Enzyme-linked immunosorbent assay (ELISA)
Caspase-1 activity assay
Quantitative real-time PCR
Table 1
Mitochondria ROS analysis
Proteome profiling analysis
Animals study and peritoneal macrophage isolation
Statistical analysis
Results
PVC-CM inhibits the inflammasome activation during macrophage-mediated inflammatory response in a paracrine fashion
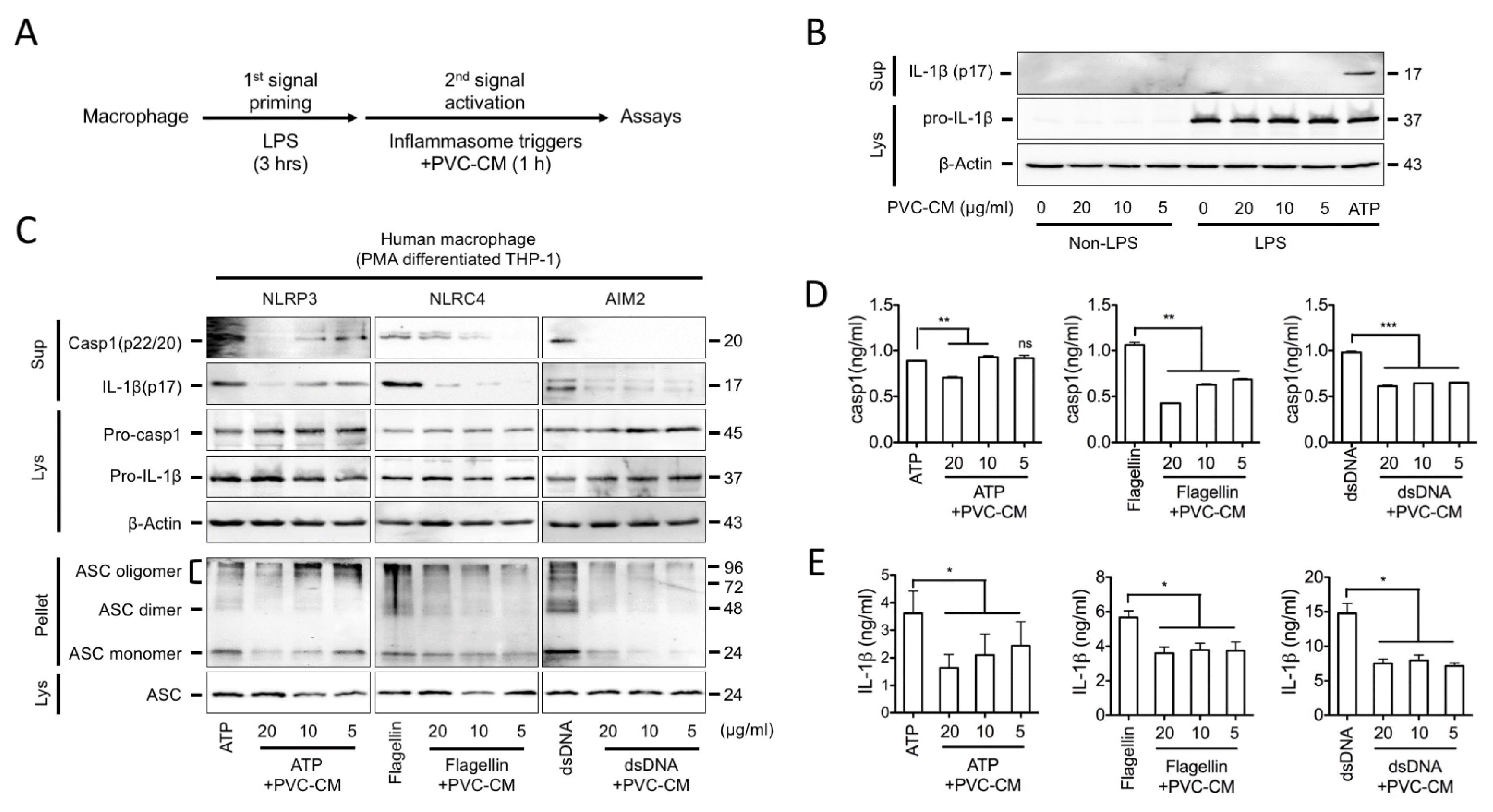 | Fig. 1The inhibitory effect of PVC-CM on inflammasome activation in human macrophage. PMA-differentiated THP-1 cells were primed with LPS (1 μg/ml) in RPMI medium containing 10% FBS and antibiotics for 3 h, followed by incubation with inflammasome activators for 1 h. (A) Schematic diagram of steps involved in the process of inflammasome activation. (B) Cells were cultured in RPMI media containing the indicated concentration of PVC-CM or ATP (2 mM) as a positive control for 1 h. IL-1β secretion was analyzed by immunoblotting. (C) PMA-differentiated THP-1 cells were treated with ATP (2 mM), flagellin (0.5 μg/ml), or dsDNA (2 μg/ml) for 1 h in the presence of PVC-CM. Cellular supernatant (Sup), lysate (Lys) and cross-linked pellets (Pellet) from whole-cell lysates were analyzed with the indicated anti-sera in an immunoblot assay. (D, E) Secreted caspase-1 and IL-1β were quantitated by an ELISA-based assay kit and the data are presented as bar graphs. Error bars indicate SD (*p<0.05, **p<0.01, ***p<0.001). |
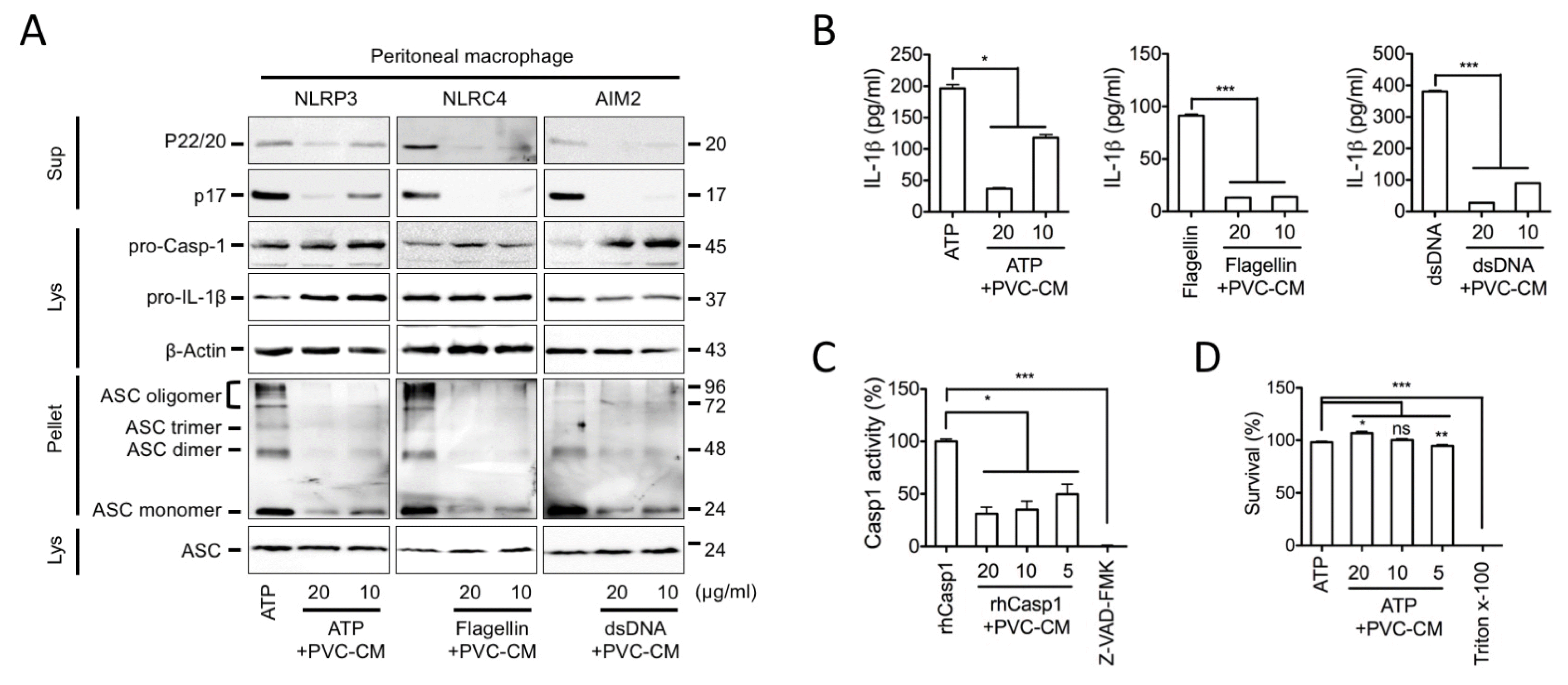 | Fig. 2PVC-CM inhibits the inflammasomes activation and caspase-1 activity in mouse peritoneal macrophage. Peritoneal macrophages were isolated, primed with LPS for 3 h, and subjected to the indicated inflammasome triggers in the present of PVC-CM. (A) Sup, Lys, and Pellet from whole-cell lysates were analyzed for caspase-1, IL-1β, or ASC pyroptosome by immunoblot assay. (B) Supernatant mouse IL-1β levels were measured by ELISA. (C) rhCasp1 was incubated with its substrate (YVAD-pNA) in the presence of PVC-CM as indicated. Relative Casp1 activity in the reaction without the rhCasp1 was set as 0% and that in the reaction with Casp1 and YVAD-pNA without PVC-CM was set as 100%. Z-VAD indicates Z-VAD-FMK, a pan-caspase inhibitor. (D) The cytotoxicity of PVC-CM was measured after applying the indicated dosage of PVC-CM to PMA-differentiated THP-1 for 1 h, identical with the inflammasome-activating step. The survival rate of the Triton-treated group was set as 0% and that of the non-treated group was set as 100%. Error bars indicate SD (*p<0.05, **p<0.01, ***p<0.001). |
PVC-CM relieves inflammation and ER stress in macrophages of MSU-induced peritonitis
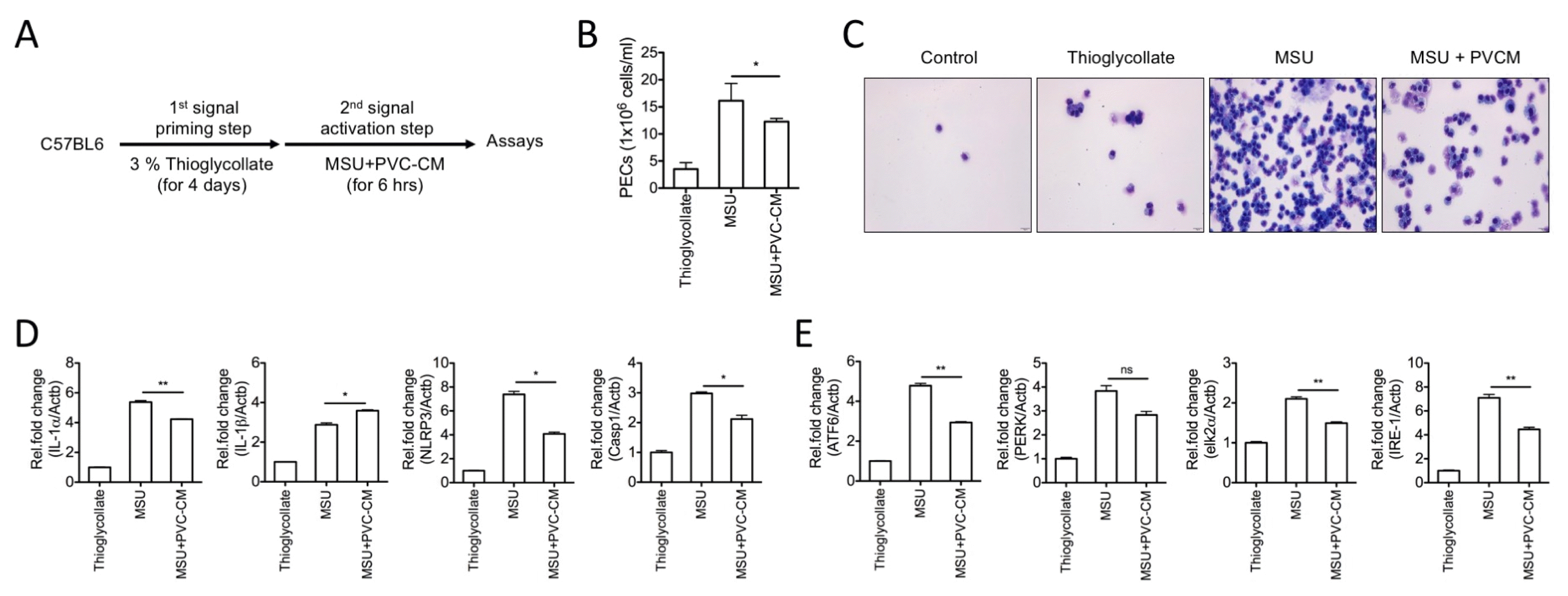 | Fig. 3PVC-CM reduces inflammation and ER stress in in macrophages of MSU-induced peritonitis. (A) Schematic diagram of experiments using the peritonitis mouse model (n=8 per group). (B) PECs were determined by counting exudate cells with a cell counter. (C) Cytospins of isolated PECs were stained with Wright-Giemsa stain. (D, E) Relative mRNA levels of inflammatory (IL-1α, IL-1β, NLRP3, and Casp1) and ER stress-related genes (ATF6, PERK, elk2α, and IRE-1) were analyzed using SYBR green-based quantitative real-time PCR. Error bars indicate SD (*p<0.05, **p<0.01). |
PVC-CM treatment reduces IL-1β secretion in human macrophages through regulation of serpin E1 and angiogenin secretion
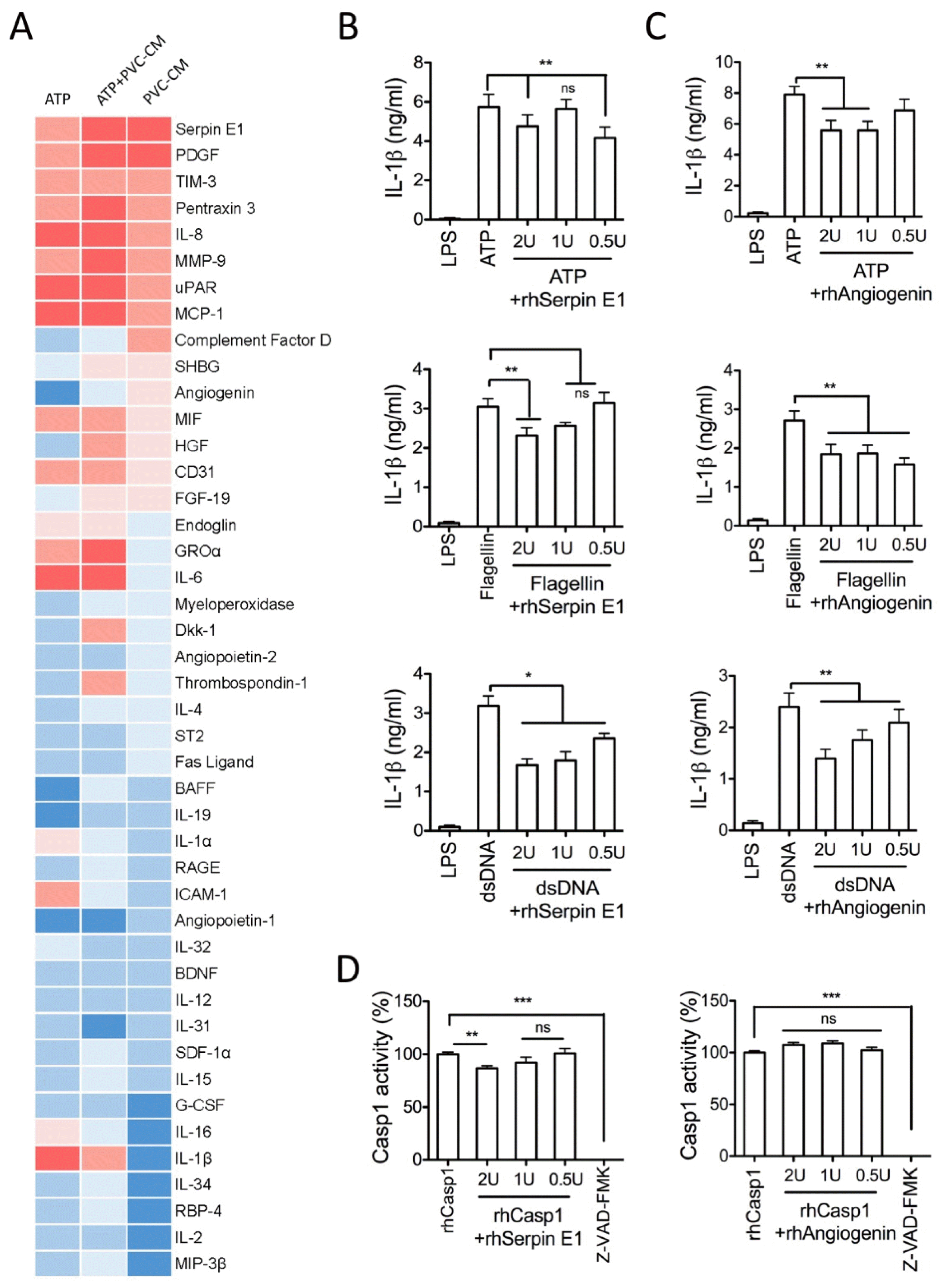 | Fig. 4Secretome analysis. Proteome profiler arrays were evaluated as described in Methods. (A) The heat map represents the subsequent image analysis and quantification of pixel intensity for each spot. (B, C) LPS-primed THP-1 cells treated with inflammasome triggers in the absence or presence of rhSerpin E1 or rhAngiogenin for 1 h. IL-1β levels in the supernatants were measured by ELISA. (D) Caspase-1 activity was determined by colorimetric assay. Error bars indicate SD (*p<0.05, **p<0.01, ***p<0.001). |
PVC-CM suppresses NLRP3 inflammasome activation through mitochondrial ROS production in macrophages
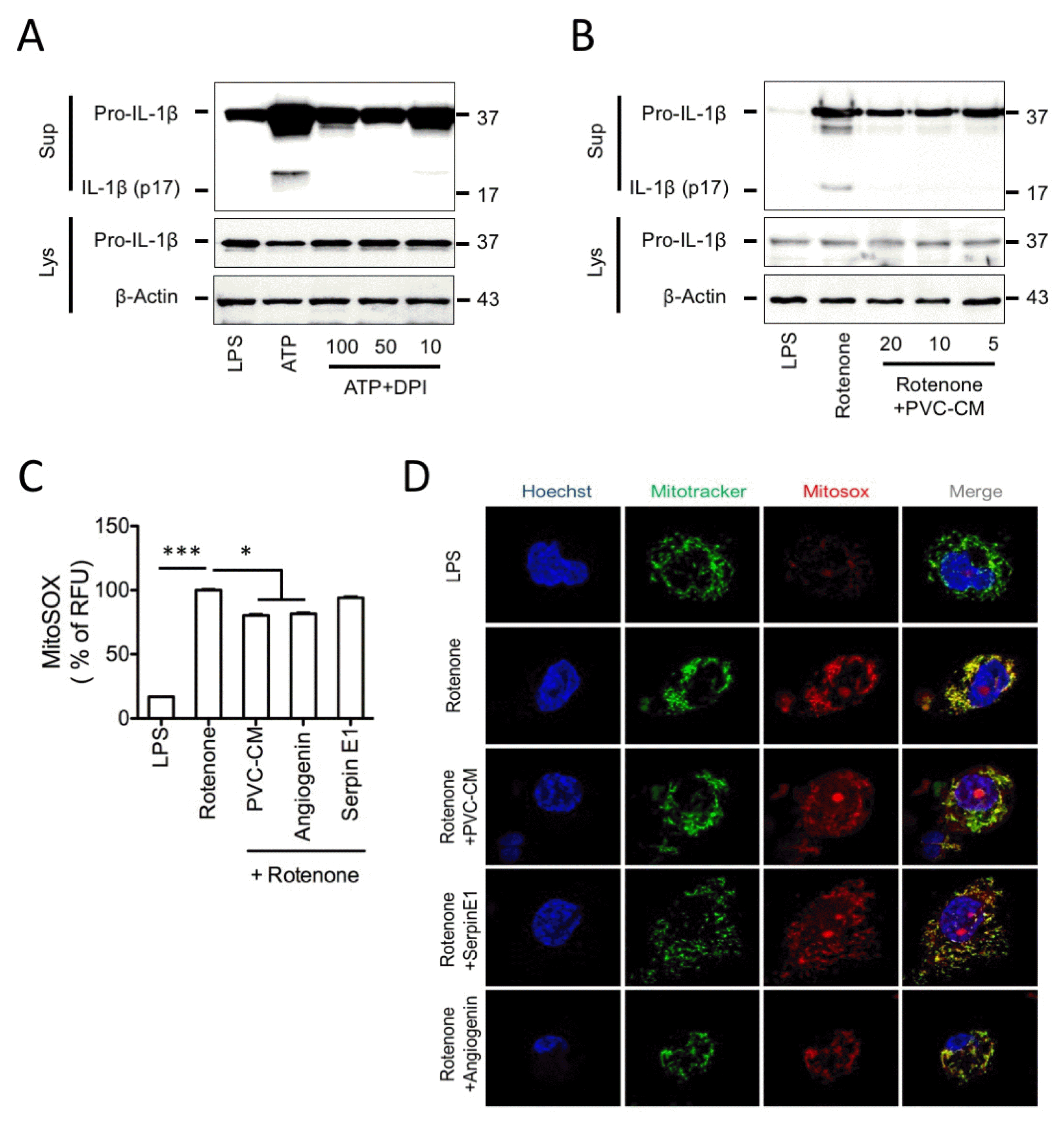 | Fig. 5The Mechanism underlying the inhibitory effect of PVC-CM on NLRP3 inflammasome via mitochondrial ROS generation. (A) LPS-primed THP-1 cells treated with ATP (2 mM) and the indicated DPI (100 to 10 μM) for 1 h. Sup and Lys were analyzed by immunoblot using the indicated anti-sera. (B) LPS-primed THP-1 cells were treated with rotenone (20 μM) and the indicated dosages of PVC-CM for 6 h, after which mitochondrial ROS generation was analyzed by immunoblot. (C) Mitochondrial ROS (MitoSOX) were quantified using a fluorometric microplate reader. (D) Relative fluorescence intensities of Mito Tracker (mitochondria) and MitoSOX (mitochondrial ROS) were analyzed using confocal microscopy. Hoechst was used for nuclear staining. Error bars indicate SD (*p<0.05, **p<0.01, ***p<0.001). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download