Abstract
The robust capacity of skeletal muscle stem cells (SkMSCs, or satellite cells) to regenerate into new muscles in vivo has offered promising therapeutic options for the treatment of degenerative muscle diseases. However, the practical use of SkMSCs to treat muscle diseases is limited, owing to their inability to expand in vitro under defined cultivation conditions without loss of engraftment efficiency. To develop an optimal cultivation condition for SkMSCs, we investigated the behavior of SkMSCs on synthetic maltose-binding protein (MBP)-fibroblast growth factor 2 (FGF2)-immobilized matrix in vitro. We found that the chemically well-defined, xeno-free MBP-FGF2-immobilized matrix effectively supports SkMSC growth without reducing their differentiation potential in vitro. Our data highlights the possible application of the MBP-FGF2 matrix for SkMSC expansion in vitro.
Skeletal muscle stem cells (SkMSCs), called as satellite cells, are the undifferentiated cells residing between the basal lamina and the plasma membrane of myofibers. These cells play significant roles in the regeneration of the damaged muscle or maintenance of skeletal muscle homeostasis (1, 2). Under normal physiological conditions, SkMSCs remain in a quiescent state; however, these cells undergo vigorous proliferation in response to muscle injury or under degenerative pathological condition through the stimulation of multiple mitogenic factors and differentiate into myocytes, which may fuse to the pre-existing myofibers or form nascent myofibers (3, 4).
The robust proliferative capacity and myogenic differentiation potential of SkMSCs have gathered interest among researchers to develop cell-based therapeutics for the treatment of degenerative muscle diseases (5). Despite significant progress in the understanding of the major signaling mechanisms to control cell proliferation and differentiation, SkMSCs show limited in vivo engraftment efficiency upon in vitro expansion (6). Furthermore, in vitro culture condition to promote initial adhesion and growth of SkMSCs require the use of Matrigel, a chemically undefined and animal-derived mixtures of extracellular matrix (7). These limitations act as a major hurdle in the development of SkMSC-based therapeutics. While several studies have shown that the manipulation of key myogenic transcription factors or signaling pathways may improve the engraftment efficiency of SkMSCs, other evidences indicate that the extracellular in vitro niche may not completely recapitulate the in vivo environment (8, 9). A biomaterial-based synthetic substrate with skeletal muscle-matching elasticity was shown to effectively support the in vitro self-renewal of SkMSCs and their subsequent in vivo engraftment within host tissues (10). Hence, the development of a well-defined synthetic niche to culture SkMSCs in vitro without any loss of their phenotypes is critically important to enhance therapeutic use (11).
We have previously developed a novel method to immobilize fibroblast growth factor 2 (FGF2) into tissue culture polystyrene substrates (12) and demonstrated that the FGF2-immobilized matrix controls the stem cell lineage commitment into targeted cell types through heparan sulfate proteoglycan (HSPG)-mediated cell adhesion behavior and the consequent cell-matrix interactions (13, 14). In this study, we extended the application of the maltose-binding protein (MBP)-FGF2 matrix to SkMSC culture. Our results demonstrate that the MBP-FGF2-immobilized matrix can support initial adhesion and activation of freshly isolated quiescent SkMSCs. In addition, the SkMSCs cultured on the MBP-FGF2 matrix can proliferate and subsequently differentiate into myotubes, similar to the SkMSCs cultured on Matrigel. Thus, our chemically well-defined and xeno-free cell culture platform provides a novel method to expand SkMSCs and may accelerate the potential use of SkMSCs for therapeutic purposes.
MBP was introduced to immobilize FGF2 into the hydrophobic surface of tissue culture polystyrene (12, 14). MBP-FGF2 fusion protein was expressed and produced from Escherichia coli K12 TB1 carrying a pMAL-FGF2 plasmid that contains human FGF2 165 cDNA. The recombinant MBP-FGF2 was prepared in phosphate-buffered saline at varying concentrations (10, 25, 30, and 50 μg/mL) and applied to the surface of cell culture vessel following incubation for 1 h at room temperature (24°C), followed by overnight incubation at 4°C. To minimize non-specific cell adhesion, the surface of the cell culture vessel was blocked with 1% bovine serum albumin for 1 h at room temperature prior to cell seeding.
All animal experimental procedures were performed according to the protocol (SCH16-0029) approved by Soonchunhyang University Animal Care and Use Committee. Quiescent SkMSCs (CD31-, CD45-, and Sca-1-negative and integrin α7-positive) were isolated from hindlimb muscles of 6- to 8-week-old ICR mice by magneticactivated cell sorting as previously described (15). The isolated quiescent SkMSCs were immediately seeded and cultured in growth medium (Ham-F10, 20% horse serum, 1% penicillin-streptomycin, 5 ng/mL of human FGF2 (PeproTech, Rocky Hill, NJ, USA) in 5% CO2 incubator at 37°C.
SkMSCs at exponential growth phase were prepared from the gastrocnemius muscle of 6- to 8-week-old ICR mice as previously described (16). SkMSCs were plated on Matrigel-coated culture dishes and cultured until 70~80% confluence in AmnioMAX-II complete medium (Thermo Fisher Scientific, Waltham, MA, USA). Cells were detached from culture plates with 0.05% trypsin and preplated for 10 min in an uncoated cell culture dish to remove fibroblasts before seeding for experiments. Myogenic differentiation was induced using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% heat-inactivated horse serum.
SkMSCs were seeded at a density of 2×103 cells/well in 96-well plates coated with either Matrigel or MBP-FGF2 and cultured in growth medium in the absence or presence of 5 ng/mL of human FGF2. Cell proliferation rate was measured using CellVia enhanced cell viability assay kit (Ab Frontier, Seoul, Korea). Briefly, 10 μL of CellVia reagent was added to each well and the cells were incubated for 1 h at 37°C in 5% CO2 atmosphere, and the absorbance of each well was measured at 450 nm using a Multi Scan GO spectrophotometer (Thermo Fisher Scientific, Finland).
Immunofluorescence staining was performed as previously described (17). Primary antibodies against myogenin (1:100, sc-52903, Santa Cruz Biotechnology, Dallas, TX, USA), MYOD (1:250, sc-377460, Santa Cruz Biotechnology), myosin heavy chain (MyHC; 0.3 μg/mL, MF20, Developmental Studies Hybridoma Bank, Iowa City, IA, USA), and vinculin (1:100, ab129002, Abcam, Cambridge, MA, USA) were used. Phalloidin-fluorescein isothiocyanate (5 μg/mL, P5282, Sigma, St. Louis, MO, USA) was used to detect F-actin. Matching secondary antibodies conjugated to Alexa Fluor 488 (1:400, A21121, Thermo Fisher Scientific) or Cy3 (1:400, A10520, Thermo Fisher Scientific; 1:500, 115-165-207, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were used. Cell nuclei were counter-stained with 4′, 6-diamidino-2-phenylindole (DAPI, Invitrogen, Waltham, MA, USA). Cell images were obtained using Nikon digital SLR camera (DS-i2) attached to Nikon eclipse Ti-U inverted microscope.
For confocal microscopy, cells were grown in Lab-Tek II eight-well chamber slide (154534, Nunc, Waltham, MA, USA) coated with either Matrigel or MBP-FGF2. Subcellular localization of vinculin and F-actin was imaged using confocal laser scanning microscopy (Zeiss LSM 710 META, Jena, Germany).
All results are presented as the mean±standard error of mean (SEM) and were tested by comparing two experimental groups using the two-tailed Student’s t-test. Graph and bar diagram were generated using GraphPad Prism software. p-values are indicated as *p≤0.05, **p≤0.01, ***p≤0.001, and p-values ≤0.05 were considered statistically significant.
Previous studies have shown that the first step in the myogenic differentiation of quiescent SkMSCs is the activation of myogenic markers, as evident from the induction of MyoD expression. As a consequence, the activated SkMSCs rapidly proliferate until being committed to enter myogenic differentiation program (18). To investigate whether MBP-FGF2-immobilized matrix could support the initial adhesion and activation of quiescent SkMSCs in a concentration-dependent manner while inhibiting their spontaneous differentiation during in vitro expansion, we examined the expression of MyoD, an indicator of activated SkMSCs, and myogenin, a marker for the onset of myogenic differentiation, in SkMSCs cultured on Matrigel or MBP-FGF2 matrix. We seeded and cultured freshly isolated SkMSCs for up to 48 h and observed a slight decrease in the number of SkMSCs that adhered to the MBP-FGF2-immobilized matrix as compared to Matrigel (data not shown). As shown in Fig. 1, the majority (approximately 98%) of the adhered SkMSCs cultured on the MBP-FGF2-immobilized matrix showed robust expression of MyoD, consistent with the observation reported for the cells cultivated on Matrigel substrate. In contrast to MyoD expression pattern, myogenin expression level remained very low (less than 2%) in the cells cultured on both Matrigel and the MBP-FGF2-immobilized matrix. These results suggest that SkMSCs grown on the MBP-FGF2-immobilized matrix efficiently undergo activation process and mostly remain in their undifferentiated states.
We examined the efficacy of the MBP-FGF2 immobilized matrix in supporting SkMSC proliferation. To determine whether the MBP-FGF2-immobilized matrix alone is sufficient to support the expansion of SkMSCs, SkMSCs were cultured on the MBP-FGF2-immobilized matrix at different concentrations in the absence of sFGF2 for 4 days. As shown in Fig. 2A, 2B, SkMSCs cultured on the MBP-FGF2 matrix showed a steady growth pattern in the absence of sFGF2 without any dose-dependent pattern; the proliferation rate of these cells was approximately 40~50% of that reported for the SkMSCs cultivated in the presence of sFGF2. In contrast, the cells cultured on BSA substrate completely failed to adhere and proliferate even in the presence of sFGF2. Therefore, the MBP-FGF2-immobilized matrix may exhibit decent mitogenic activity.
We evaluated the proliferative capacity of the SkMSCs cultured on the MBP-FGF2-immobilized matrix with varying concentrations in the presence of sFGF2. SkMSCs cultured on Matrigel-coated substrates in the presence of sFGF2 exhibited a robust proliferation profile during 4 days of culture (Fig. 2C). SkMSCs cultured on the MBP-FGF2-immobilized matrix also showed strong proliferation ability in a dose-independent manner, but their proliferation was slightly slower than that observed for the cells cultured on Matrigel-coated substrate. Considering that lesser number of SkMSCs were adhered to the MBP-FGF2-immobilized matrix at the beginning of culture, our results suggest that SkMSCs, once attached to the matrix, may competently proliferate. Taken together, we conclude that the MBP-FGF2-immobilized matrix effectively supports SkMSC proliferation and the addition of sFGF2 to the medium may synergistically enhance SkMSC proliferation.
To determine whether SkMSCs cultured on the MBP-FGF2-immobilized matrix maintain myogenic differentiation potential, we measured myogenic differentiation and cell fusion indices in SkMSCs expanded on MBP-FGF2-immobilized matrix. Freshly isolated SkMSCs were expanded using both Matrigel and MBP-FGF2-immobilized matrix. The cells were reseeded on Matrigel-coated cell culture vessels in differentiation medium and immediately subjected to myogenic differentiation. After 3 days of differentiation, the cells were immunostained for sarcomeric MyHC expression.
As shown in Fig. 3A, SkMSCs precultured on the MBP-FGF2-immobilized matrix were able to differentiate into myotubes with an efficiency comparable to that of the cells precultured on Matrigel. Very similar differentiation indices were observed for both Matrigel- and MBP-FGF2 matrix-precultured cells (Fig. 3B). We noticed an interesting but not significant gradual increment in differentiation indices for the MBP-FGF2 matrix-precultured cells in a dose-dependent manner. Cell fusion indices showed no significant differences between Matrigel- and MBP-FGF2-precultured cells (Fig. 3C). Similar to differentiation indices, a minor increase in the fused myotube population was observed in the cells cultured on the matrix with high dose of MBP-FGF2. We do not know the nature of this mild effect, and further studies are warranted to determine whether SkMSCs precultured on the MBP-FGF2 matrix become more myogenic. Thus, the SkMSCs that expanded on the MBP-FGF2-immobilized matrix maintain robust myogenic potential equivalent to that of the cells precultured on Matrigel.
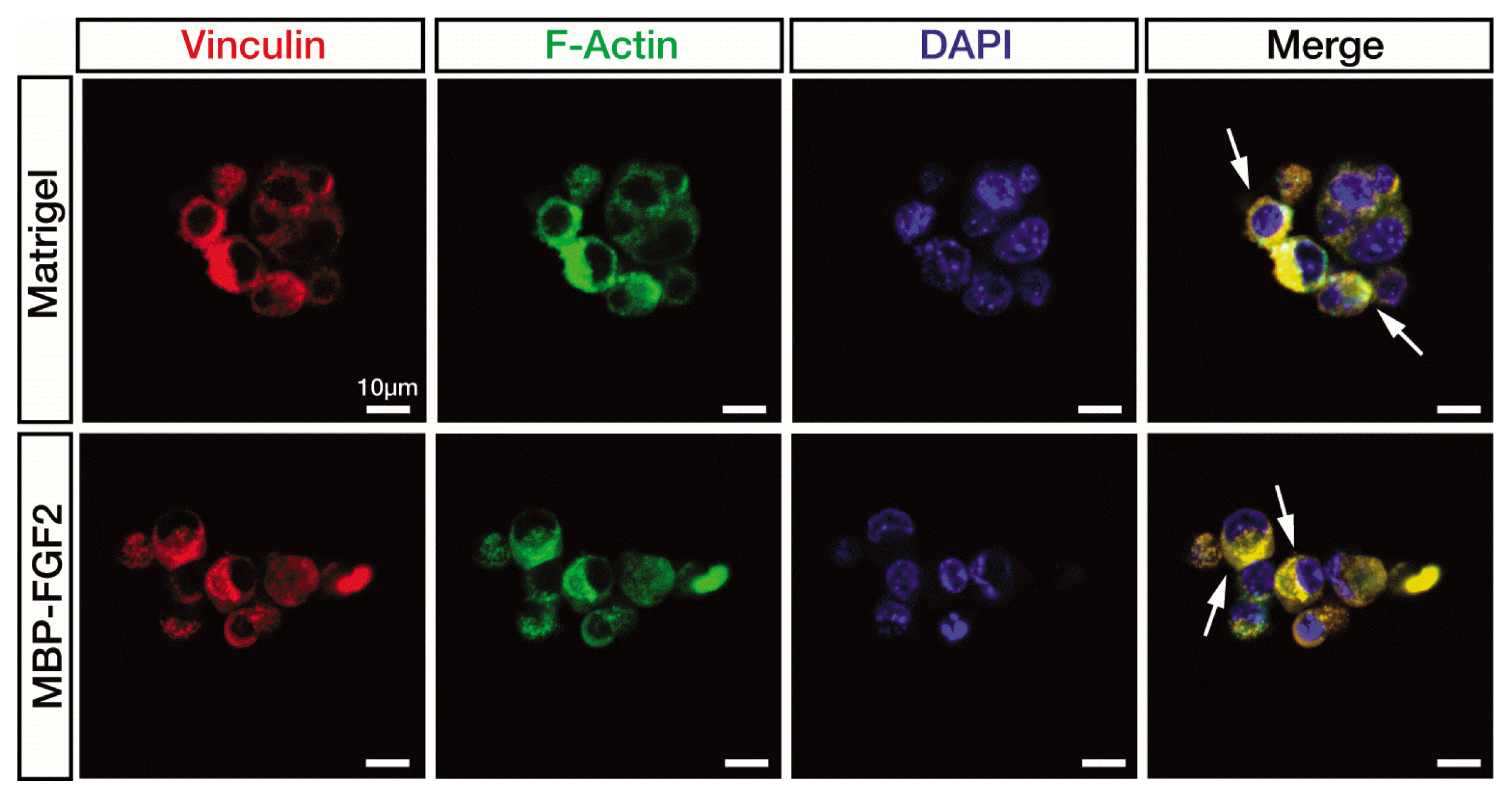
Extracellular matrix is connected to the intracellular cytoskeleton through large integrin-based multi-protein complexes called FAs, which control cell morphology, migration, and adhesion (19). Vinculin is involved in anchoring F-actin to the cell membrane through its binding with talin-integrin complex, leading to integrin clustering (20). To determine how FAs are distributed within the SkMSCs adhered to the immobilized MBP-FGF2, the expression of vinculin and F-actin was analyzed with immunofluorescence staining, followed by confocal microscopy. As shown in Fig. 4, vinculin expression was evenly distributed around the nucleus in the cells cultured on the MBP-FGF2-immobilized matrix and no significant difference in vinculin expression pattern was observed between these cells and those cultured on Matrigel. In both cases, vinculin expression was mostly co-localized with actin cytoskeleton. These results together with our previous findings (12, 13) suggest that HSPG on the surface of SkMSCs binds to the FGF2 moiety within the MBP-FGF2-immobilized matrix.
In conclusion, the present study demonstrates that the MBP-FGF2-immobilized matrix may serve as a chemically well-defined and xeno-free cell culture substrate to support the adhesion, activation, and proliferation of SkMSCs and maintain their in vitro myogenic differentiation at the level comparable to that observed with the conventionally used Matrigel substrate. We failed to observe any significant dose-dependent difference with MBP-FGF2 concentrations ranging from 10 to 50 μg/mL. We speculate that the immobilized MBP-FGF2 at 10 μg/mL of concentration is sufficient enough to support SkMSC behavior. The present study provides a proof-of-principle that a chemically well-defined, xeno-free, easy-to-use, and cost-effective platform for the expansion of SkMSCs without the loss of their self-renewal and myogenic potential may be achieved with the MBP-FGF2-immobilized matrix. Currently, we are further improving the biofunctionality of this synthetic matrix by complexing with other chemically defined, xeno-free adhesion molecule(s) to improve initial cell attachment and proliferation of SkMSCs. Additionally, future engraftment experiments using SkMSCs expanded on the MBP-FGF2-immobilized matrix are warranted.
Acknowledgments
This research was supported by the Global Research Development Program grant (2016K1A4A3914725) and research grant (2016R1A2B4012956) from the National Research Foundation of Korea (NRF) to JK Yoon and the research grant (HI17C1193) from Korea Health Industry Development Institute (KHIDI) to Y Hwang.
References
1. Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003; 35:1151–1156. DOI: 10.1016/S1357-2725(03)00042-6. PMID: 12757751.

2. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013; 93:23–67. DOI: 10.1152/physrev.00043.2011. PMID: 23303905. PMCID: PMC4073943.

3. Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003; 203:89–99. DOI: 10.1046/j.1469-7580.2003.00195.x. PMID: 12892408. PMCID: PMC1571137.

4. Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev Biol. 2000; 218:115–124. DOI: 10.1006/dbio.1999.9565. PMID: 10656756.

5. Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008; 14:82–91. DOI: 10.1016/j.molmed.2007.12.004. PMID: 18218339.

6. Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, Rando TA. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Reports. 2015; 5:621–632. DOI: 10.1016/j.stemcr.2015.08.004. PMID: 26344908. PMCID: PMC4624935.

7. Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015; 56:1–8. DOI: 10.3109/03008207.2014.947369. PMID: 25047058. PMCID: PMC4464813.

8. Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012; 30:232–242. DOI: 10.1002/stem.773. PMID: 22045613. PMCID: PMC3384696.

9. Boldrin L, Neal A, Zammit PS, Muntoni F, Morgan JE. Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated. Stem Cells. 2012; 30:1971–1984. DOI: 10.1002/stem.1158. PMID: 22730231. PMCID: PMC3465801.

10. Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010; 329:1078–1081. DOI: 10.1126/science.1191035. PMID: 20647425. PMCID: PMC2929271.

11. Han WM, Anderson SE, Mohiuddin M, Barros D, Nakhai SA, Shin E, Amaral IF, Pêgo AP, García AJ, Jang YC. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci Adv. 2018; 4:eaar4008. DOI: 10.1126/sciadv.aar4008. PMID: 30116776. PMCID: PMC6093653.

12. Kang JM, Han M, Park IS, Jung Y, Kim SH, Kim SH. Adhesion and differentiation of adipose-derived stem cells on a substrate with immobilized fibroblast growth factor. Acta Biomater. 2012; 8:1759–1767. DOI: 10.1016/j.actbio.2012.01.005. PMID: 22285427.

13. Kang J, Park HM, Kim YW, Kim YH, Varghese S, Seok HK, Kim YG, Kim SH. Control of mesenchymal stem cell phenotype and differentiation depending on cell adhesion mechanism. Eur Cell Mater. 2014; 28:387–403. DOI: 10.22203/eCM.v028a27. PMID: 25422949.

14. Kim JH, Park Y, Jung Y, Kim SH, Kim SH. Combinatorial therapy with three-dimensionally cultured adipose-derived stromal cells and self-assembling peptides to enhance angiogenesis and preserve cardiac function in infarcted hearts. J Tissue Eng Regen Med. 2017; 11:2816–2827. DOI: 10.1002/term.2181. PMID: 27256923.

15. Motohashi N, Asakura Y, Asakura A. Isolation, culture, and transplantation of muscle satellite cells. J Vis Exp. 2014; Apr. 8. [Epub]. DOI: 10.3791/50846. PMID: 24747722. PMCID: PMC4131689.

16. Michailovici I, Harrington HA, Azogui HH, Yahalom-Ronen Y, Plotnikov A, Ching S, Stumpf MP, Klein OD, Seger R, Tzahor E. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development. 2014; 141:2611–2620. DOI: 10.1242/dev.107078. PMID: 24924195. PMCID: PMC4067960.

17. Han XH, Jin YR, Seto M, Yoon JK. A WNT/beta-catenin signaling activator, R-spondin, plays positive regulatory roles during skeletal myogenesis. J Biol Chem. 2011; 286:10649–10659. DOI: 10.1074/jbc.M110.169391. PMID: 21252233. PMCID: PMC3060516.

18. Asfour HA, Allouh MZ, Said RS. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018; 243:118–128. DOI: 10.1177/1535370217749494. PMID: 29307280. PMCID: PMC5788151.

19. Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol. 2007; 179:1043–1057. DOI: 10.1083/jcb.200703036. PMID: 18056416. PMCID: PMC2099183.

20. Atherton P, Stutchbury B, Jethwa D, Ballestrem C. Mechanosensitive components of integrin adhesions: role of vinculin. Exp Cell Res. 2016; 343:21–27. DOI: 10.1016/j.yexcr.2015.11.017. PMID: 26607713. PMCID: PMC4856733.

Fig. 1
MBP-FGF2-immobilized matrix can support the activation of SkMSCs. (A) Immunofluorescence staining images for the expression of myogenic markers, MyoD and myogenin (MYOG), in quiescent SkMSCs grown on Matrigel or MBP-FGF2 matrix for 48 h in growth medium. Cell nuclei were counterstained with DAPI. Scale bar, 50 μm. (B) Quantitation of MyoD- and MYOG-positive cells. Marker-positive cells are presented as the percentage of total cell number from triplicate samples. Error bars indicate standard error of the mean (SEM). NS, no statistical significance.
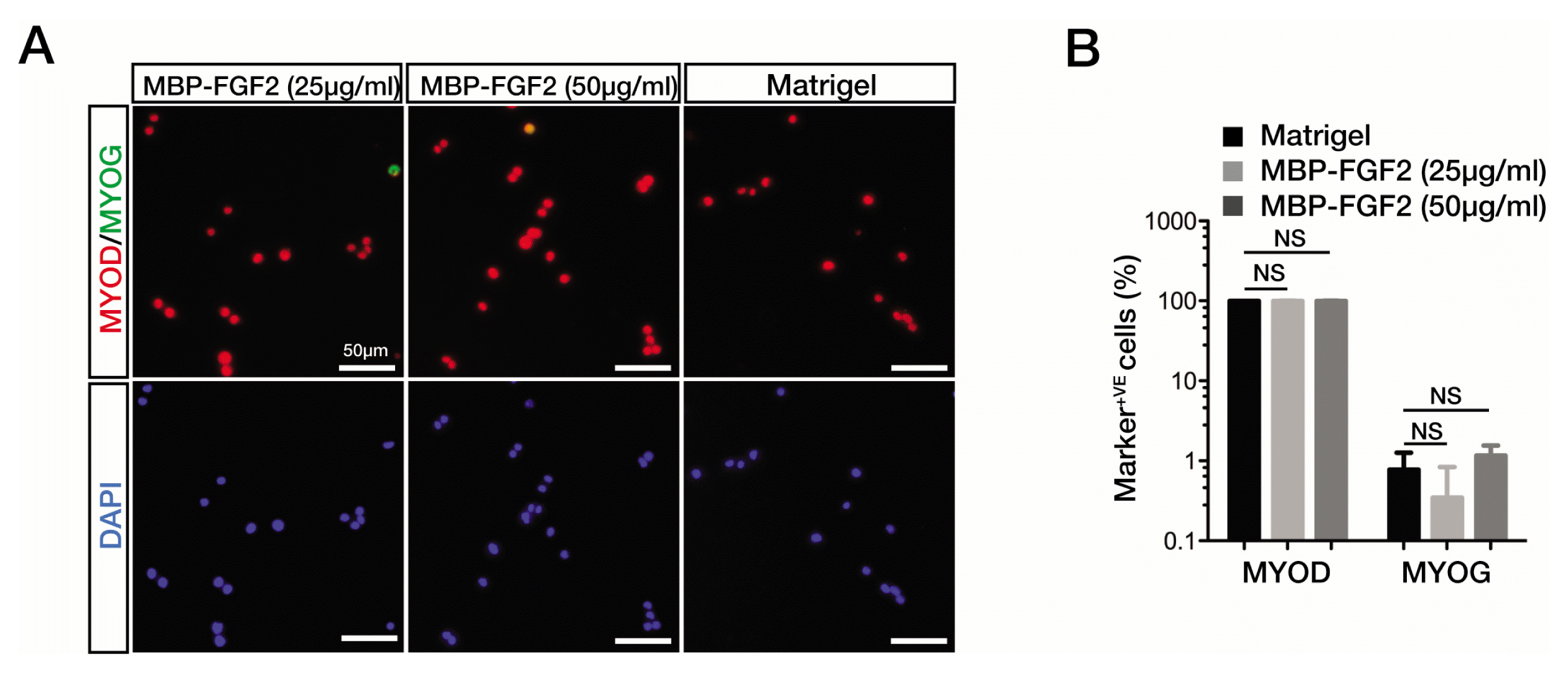
Fig. 2
SkMSCs competently proliferate on the MBP-FGF2-immobilized matrix. (A) Phase-contrast microscopic images of proliferating SkMSCs cultured on Matrigel or MBP-FGF2 matrix-coated surface with or without FGF2 supplementation in the growth medium for 4 days. Scale bar, 50 μm. (B, C) Determination of live SkMSC density with a modified MTS assay. The MTS assay was performed daily during the time course of 4 days. SkMSCs were cultured on BSA-, MBP-FGF2-, or Matrigel-coated 96-well plates in the absence (B) or presence (C) of soluble FGF2 (5 ng/mL) as indicated. Viable cell density was determined from the optical density (O.D.) at 450 nm wavelength. Experiments were performed in triplicates. Data represent normalized mean values with SEM.
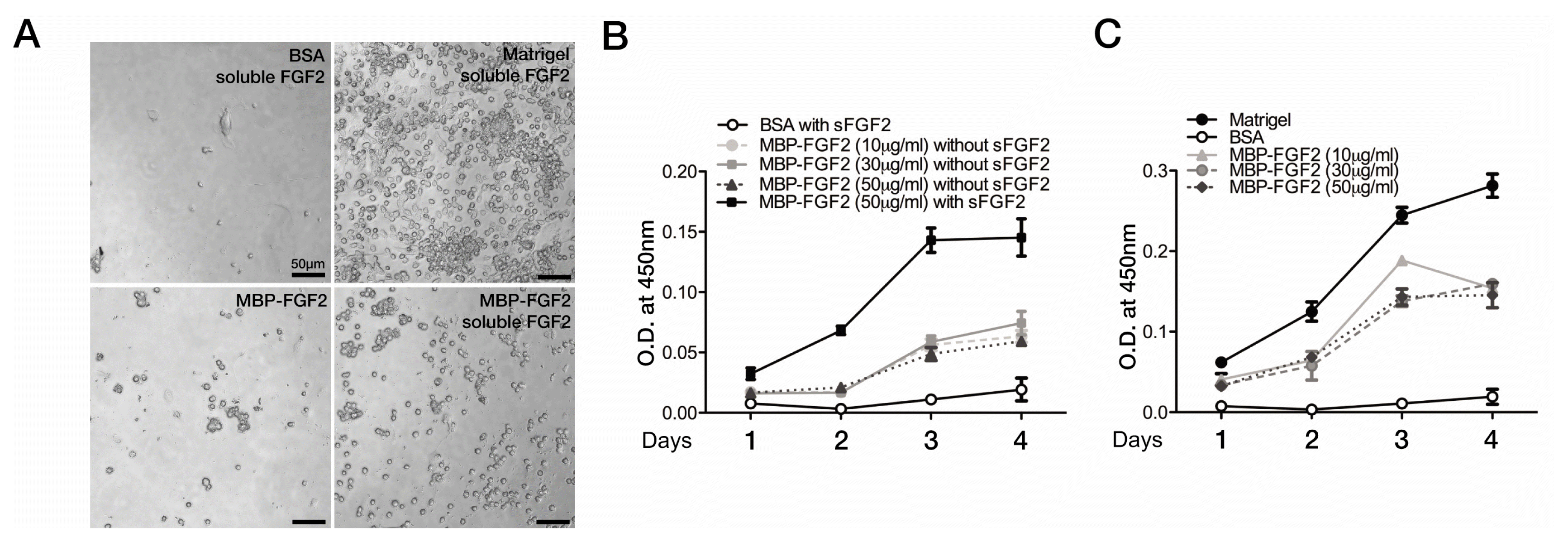
Fig. 3
SkMSCs maintain robust myogenic potential on MBP-FGF2-mmobilized matrix. (A) Immunofluorescence staining images for MyHC expression in the SkMSCs cultured on Matrigel- or MBP-FGF2 (50 μg/mL)-coated surfaces at differentiation day 3. Cell nuclei were counterstained with DAPI. Scale bar, 50 μm. (B) To calculate differentiation indices after 3 days, MyHC-positive cell nuclei were counted and expressed as the percentage of total cell nuclei. (C) Fusion indices were shown as a distribution of MyHC-positive cells based on cell nucleus number; mono, 2~5, and more than 5 nuclei. Experiments were performed in triplicates. Error bars indicate SEM. NS, no statistical significance.
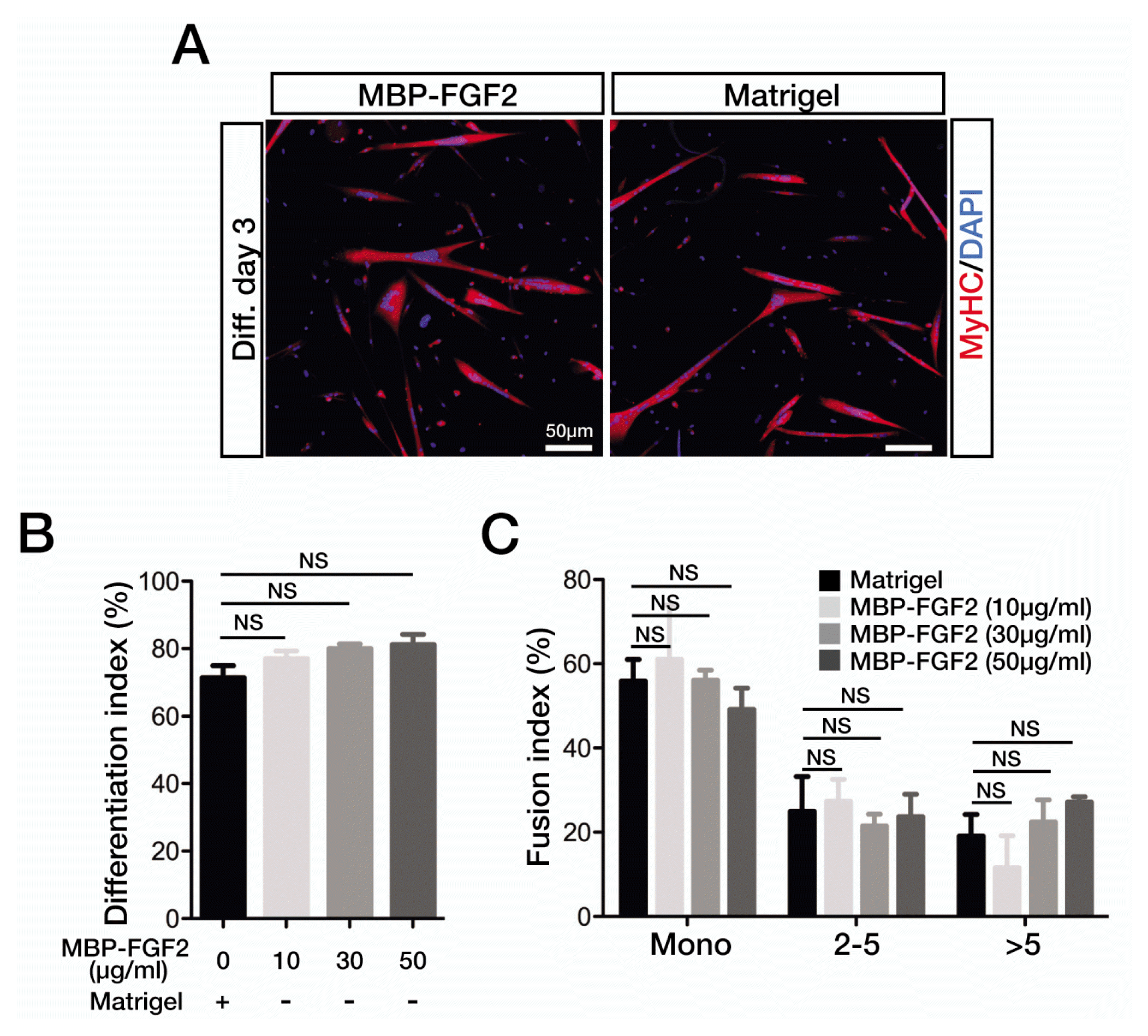
Fig. 4
Focal adhesion kinase expression is detected in the SkMSCs cultured on the MBP-FGF2-immobilized matrix. Confocal microscopic images of immunofluorescence staining of vinculin (red) and F-actin (green). SkMSCs were cultured on MBP-GF2 matrix (50 μg/mL) and Matrigel for 48 h in growth medium. White arrows indicate the cytoplasmic area overlapped between vinculin and F-actin expression. Scale bar, 10 μm.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download