Introduction
Materials and Methods
Animals
Bone marrow cell isolation and culture
Mouse
Rabbit
Peripheral blood cell isolation and culture
Preparation of cell-free ECM from cultured bone marrow MSCs
Colony-forming unit-fibroblast (CFU-F) assay
Bromodeoxyuridine (BrdU) cell proliferation assay
Fluorescence activated cell sorter (FACS) analysis
Differentiation potential
Bone differentiation
Adipose differentiation
Rabbit chondrogenic differentiation
RT-PCR
Table 1
In vivo transplantation
PI3 kinase assay
Statistical analyses
Results
Colony-forming unit-fibroblast (CFU-F) assay and proliferation of BM MSCs and PB MSCs
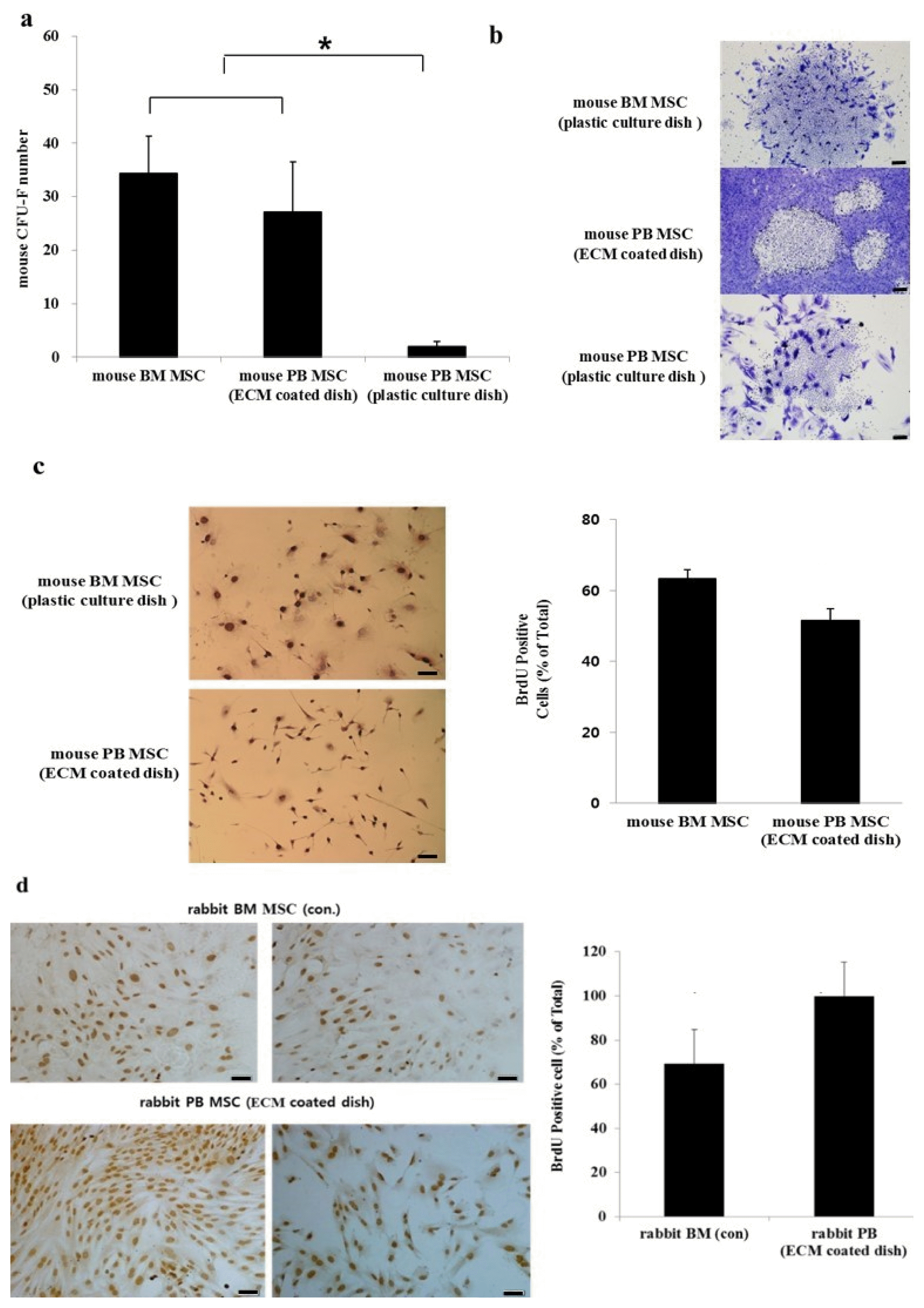 | Fig. 1CFU-F and proliferation of BM MSCs and PB MSCs. (a) Mouse BM MSCs seeded on the normal culture dish showed substantial proliferation whereas mouse PB MSCs seeded on the same kind of dish produced significantly fewer CFU-Fs (*p<0.05). There was a significant increase in the number of mouse PB MSCs plated on ECM-coated culture dish compared with mouse PB MSCs plated on a normal dish (*p<0.05). (b) Analysis of colony morphology of three dishes at ×40 magnification revealed that BM MSCs adhere to each other in contrast to the dispersed nature of mouse PB MSCs in culture. (c) Self-renewal capacity of mouse BM MSCs and mouse PB MSCs and morphology of these cells (×200 magnification). The proliferation of mouse BM MSCs and mouse PB MSCs measured by BrdU incorporation were similar (*p>0.05). Data were obtained from the mean±SE of nine fields. (d) Self-renewal of rabbit BM MSCs and rabbit PB MSCs. The proliferation of rBM MSCs and rPB MSCs measured by BrdU incorporation. Analysis of variance (**p<0.01). Scale bars=50 μM. |
Flow cytometric analysis
 | Fig. 2FACS analysis. (a) Flow cytometric analysis of the expression of mouse markers related to stem cells such as MSCs and hematopoietic stem cells. (b) Flow cytometric analysis of the expression of rabbit cell markers related to stem cells such as MSCs and hematopoietic stem cells. Data show mean±SE of three independent experiments (*p>0.05). |
Multipotent differentiation
 | Fig. 3Osteogenic and adipogenic differentiation of mouse BM MSCs and mouse PB MSCs in vitro. (a) Calcium accumulation revealed by alizarin red S staining at ×200 magnification. (b) Expression of RUNX-2, OCN, and ALP, which induce osteoblast differentiation, in both experimental groups. (c) Percentage of mineralized area/total area of the dish. Data show mean±SE of three dishes (*p<0.05). (d) Lipid droplets revealed by Oil red O staining at ×200 magnification indicating that the MSCs are capable of forming Oil red O-positive cells. (e) RT-PCR showing positive gene expression profiles related to adipogenic differentiation in induced cultures compared with uninduced (un) cultures. (f) A number of cells staining positive/total number of cells. Data show mean±SE of five fields (*p>0.05). |
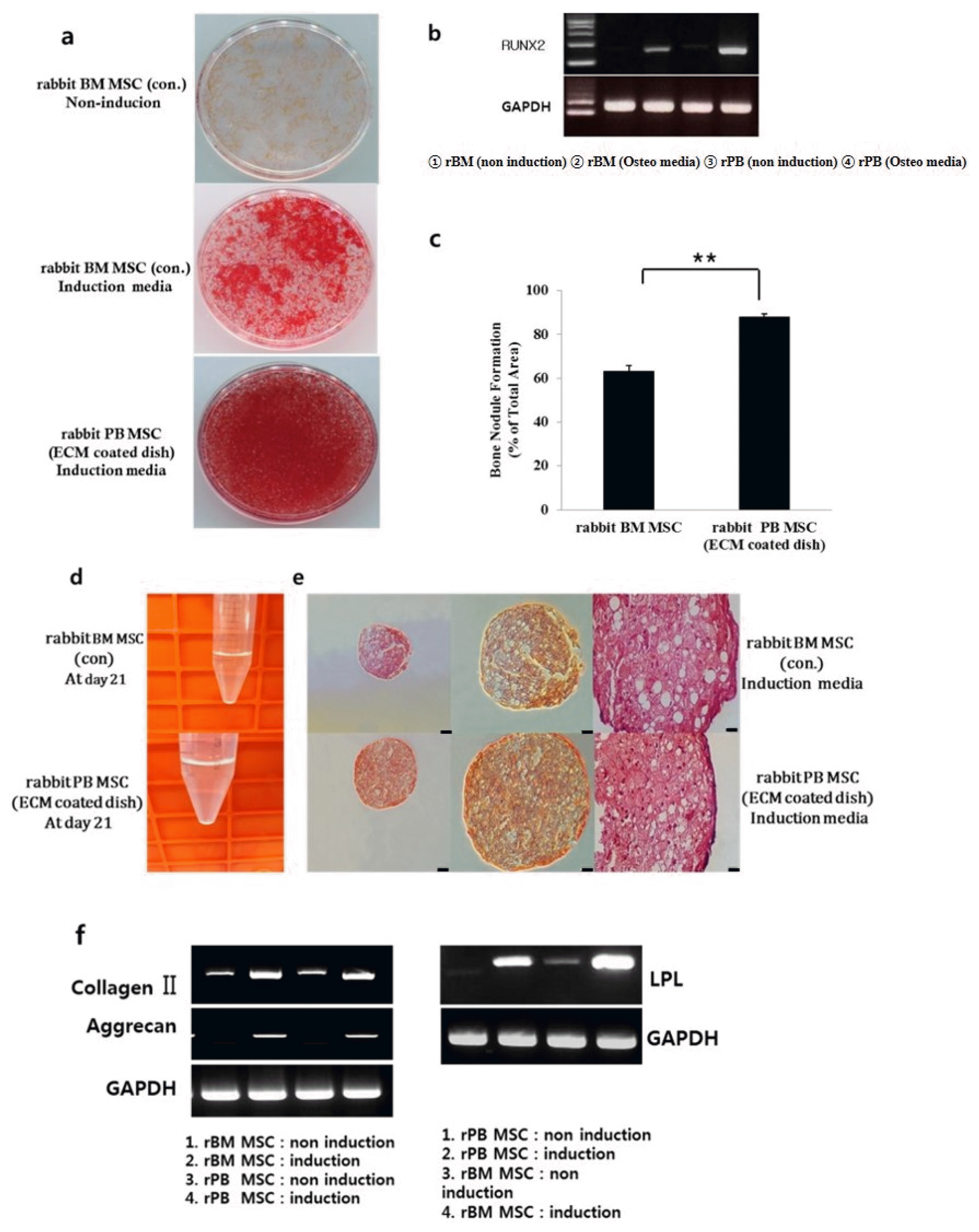 | Fig. 4Osteogenic and Chondrogenic differentiation of rabbit BM MSCs and rabbit PB MSCs in vitro. (a) Calcium accumulation revealed by alizarin red S staining at ×200 magnification. (b) Expression of RUNX-2, which induces osteoblast differentiation, in both experimental groups. (c) Percentage of mineralized area/total area of the dish. Data show mean±SE of three dishes (*p<0.05) (d) Aggregate pellet culture. Aliquots of rabbit BM MSCs and rabbit PB MSCs form a spherical pellet in 21 days. (e) Histologic sections were stained with Safranin-O. The stained images are presented as a whole sample (first columns, ×100) and at high magnification (second and last columns, ×200, ×400). (f) Total RNA was isolated from rabbit BM MSCs and rabbit PB MSCs and expression levels of type II collagen, aggrecan, and GAPDH were examined by RT-PCR analysis. |
In vivo transplantation
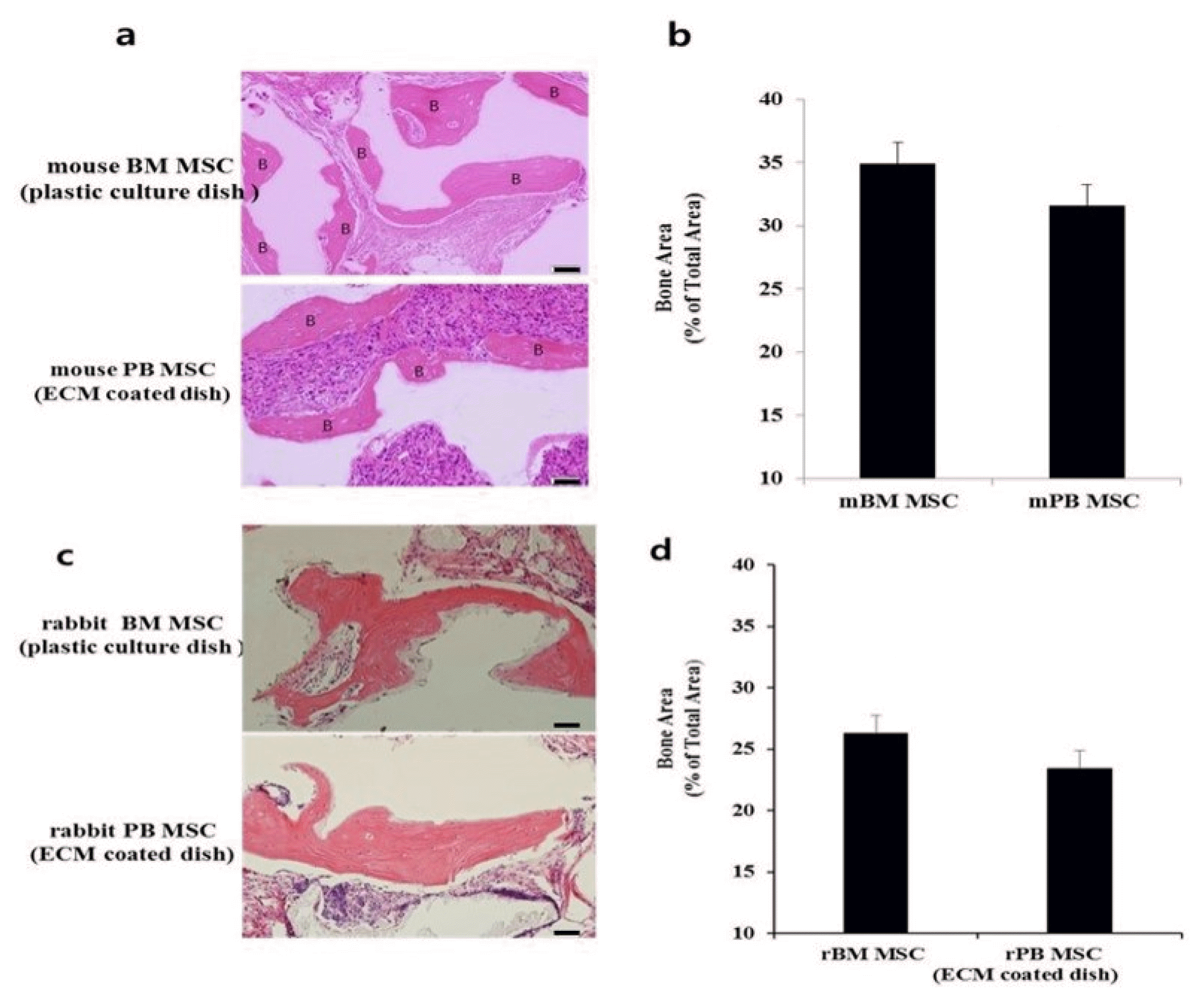 | Fig. 5Bone formation of BM MSCs and PB MSCs in vivo after transplantation into immunocompromised mice. (a, c) Bone formation by in vivo transplantation of mouse and rabbit MSCs. After 8 weeks, the transplants were harvested and sections were stained with H&E to evaluate bone formation. Scale bar=50 μm. (b, d) PB MSCs generate as much bone area as BM MSCs (*p>0.05). Data show mean±SE of bone area. |
Effect of PI3 kinase on mesenchymal stem cell attachment
 | Fig. 6PI3K modulates the number of adherent colony-forming unit-fibroblasts (CFU-Fs) in primary bone marrow cultures. (a) Hypothetical model showing the culture of mouse BM MSCs on culture dish for 16 days, before treatment with the PI3K inhibitors LY294002 (20 μM and 200 μM) and Wortmannin (10 μM and 100 μM) for 10 min and 30 min. The colony forming efficiency of cells from suspension was determined. (b, c) Mouse BM MSCs, mouse BM matrix cells, and mouse PB cells were treated with inhibitors at various concentrations and cell number was determined by trypan blue assay. Left panel: Colony forming assay of mouse BM MSCs in suspension after culture for 16 days and treatment with LY294002. Right panel: Colony forming assay of mouse BM MSCs in suspension after culture for 16 days and treatment with Wortmannin. Wortmannin treatment increased the number of cells in the supernatant in a dose- and time-dependent manner. Data are presented as means±SD. Statistical analysis was performed using Student’s t-tests. *Indicates significant difference versus control (*p<0.05; **p<0.01; ***p<0.001 by Student’s t-test). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download