1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. DOI:
10.3322/caac.21492. PMID:
30207593.

2. Li J, Siegel DA, King JB. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004–2014. Ann Epidemiol. 2018; 28:328–330. DOI:
10.1016/j.annepidem.2018.03.001. PMID:
29678312. PMCID:
PMC6080305.

3. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouvière O, Schoots IG, Wiegel T, Cornford P. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–629. DOI:
10.1016/j.eururo.2016.08.003. PMID:
27568654.

4. Kang DH, Lee JY, Park SY, Moon HS, Jeong TY, Yoo TK, Choi HY, Park HY, Lee TY, Lee SW. Efficacy and safety of tadalafil 5 mg administered once daily in Korean men with erectile dysfunction: a prospective, multicenter study. Korean J Urol. 2010; 51:647–652. DOI:
10.4111/kju.2010.51.9.647. PMID:
20856651. PMCID:
PMC2941815.

5. Fode M, Ohl DA, Ralph D, Sønksen J. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013; 112:998–1008. PMID:
23826962.

6. Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2018; PMID:
29631980.

7. Gur S, Abdel-Mageed AB, Sikka SC, Hellstrom WJG. Advances in stem cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2018; 18:1137–1150. DOI:
10.1080/14712598.2018.1534955. PMID:
30301368.

10. Peak TC, Anaissie J, Hellstrom WJ. Current perspectives on stem cell therapy for erectile dysfunction. Sex Med Rev. 2016; 4:247–256. DOI:
10.1016/j.sxmr.2016.02.003. PMID:
27871958.

11. Xin ZC, Xu YD, Lin G, Lue TF, Guo YL. Recruiting endogenous stem cells: a novel therapeutic approach for erectile dysfunction. Asian J Androl. 2016; 18:10–15. DOI:
10.4103/1008-682X.150040. PMID:
25926601. PMCID:
PMC4736335.

12. Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016; 2016:6737345. DOI:
10.1155/2016/6737345. PMID:
27057174. PMCID:
PMC4761677.

13. Hou QL, Ge MY, Zhang CD, Tian DD, Wang LK, Tian HZ, Wang WH, Zhang WD. Adipose tissue-derived stem cell therapy for erectile dysfunction in rats: a systematic review and meta-analysis. Int Urol Nephrol. 2017; 49:1127–1137. DOI:
10.1007/s11255-017-1590-2. PMID:
28417342.

14. Shan H, Chen F, Zhang T, He S, Xu L, Wei A. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS One. 2015; 10:e0121428. DOI:
10.1371/journal.pone.0121428. PMID:
25860455. PMCID:
PMC4393097.

16. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014; 14:43. DOI:
10.1186/1471-2288-14-43. PMID:
24667063. PMCID:
PMC4230647.

18. Fandel TM, Albersen M, Lin G, Qiu X, Ning H, Banie L, Lue TF, Lin CS. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012; 61:201–210. DOI:
10.1016/j.eururo.2011.07.061. PMID:
21824718. PMCID:
PMC3226917.

19. Jeon SH, Shrestha KR, Kim RY, Jung AR, Park YH, Kwon O, Kim GE, Kim SH, Kim KH, Lee JY. Combination therapy using human adipose-derived stem cells on the cavernous nerve and low-energy shockwaves on the corpus cavernosum in a rat model of post-prostatectomy erectile dysfunction. Urology. 2016; 88:226.e221–e229. DOI:
10.1016/j.urology.2015.10.021. PMID:
26522972.

20. Jeong HH, Piao S, Ha JN, Kim IG, Oh SH, Lee JH, Cho HJ, Hong SH, Kim SW, Lee JY. Combined therapeutic effect of udenafil and adipose-derived stem cell (ADSC)/brain-derived neurotrophic factor (BDNF)-membrane system in a rat model of cavernous nerve injury. Urology. 2013; 81:1108.e1107–e1114. DOI:
10.1016/j.urology.2013.01.022. PMID:
23522997.

21. Kim IG, Piao S, Lee JY, Hong SH, Hwang TK, Kim SW, Kim CS, Ra JC, Noh I, Lee JY. Effect of an adipose-derived stem cell and nerve growth factor-incorporated hydrogel on recovery of erectile function in a rat model of cavernous nerve injury. Tissue Eng Part A. 2013; 19:14–23. DOI:
10.1089/ten.tea.2011.0654. PMID:
22834730. PMCID:
PMC3530933.

22. Lee SH, Kim IG, Jung AR, Shrestha KR, Lee JH, Park KD, Chung BH, Kim SW, Kim KH, Lee JY. Combined effects of brain-derived neurotrophic factor immobilized poly-lactic-co-glycolic acid membrane with human adipose-derived stem cells and basic fibroblast growth factor hydrogel on recovery of erectile dysfunction. Tissue Eng Part A. 2014; 20:2446–2454. DOI:
10.1089/ten.tea.2013.0495. PMID:
24673637. PMCID:
PMC4161192.

23. Piao S, Kim IG, Lee JY, Hong SH, Kim SW, Hwang TK, Oh SH, Lee JH, Ra JC, Lee JY. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012; 9:1968–1979. DOI:
10.1111/j.1743-6109.2012.02760.x. PMID:
22642440.

24. You D, Jang MJ, Lee J, Suh N, Jeong IG, Sohn DW, Kim SW, Ahn TY, Kim CS. Comparative analysis of periprostatic implantation and intracavernosal injection of human adipose tissue-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Prostate. 2013; 73:278–286. DOI:
10.1002/pros.22567. PMID:
22821215.

25. Zheng T, Zhang TB, Wang CL, Zhang WX, Jia DH, Yang F, Sun YY, Ding XJ, Wang R. Icariside II promotes the differentiation of adipose tissue-derived stem cells to Schwann cells to preserve erectile function after cavernous nerve injury. Mol Cells. 2018; 41:553–561. PMID:
29902838. PMCID:
PMC6030246.
26. Ying C, Yang M, Zheng X, Hu W, Wang X. Effects of intracavernous injection of adipose-derived stem cells on cavernous nerve regeneration in a rat model. Cell Mol Neurobiol. 2013; 33:233–240. DOI:
10.1007/s10571-012-9890-7. PMID:
23161147.

27. Xu Y, Guan R, Lei H, Li H, Wang L, Gao Z, Song W, Xin Z. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med. 2014; 11:2439–2448. DOI:
10.1111/jsm.12636. PMID:
25042722.

28. Ying C, Hu W, Cheng B, Yang M, Zheng X, Wang X. Erectile function restoration after repair of resected cavernous nerves by adipose-derived stem cells combined with autologous vein graft in rats. Cell Mol Neurobiol. 2014; 34:393–402. DOI:
10.1007/s10571-013-0024-7. PMID:
24398902.

29. Yang R, Fang F, Wang J, Guo H. Adipose-derived stem cells ameliorate erectile dysfunction after cavernous nerve cryoinjury. Andrology. 2015; 3:694–701. DOI:
10.1111/andr.12047. PMID:
26198799.

30. Chen X, Yang Q, Zheng T, Bian J, Sun X, Shi Y, Liang X, Gao G, Liu G, Deng C. Neurotrophic effect of adipose tissue-derived stem cells on erectile function recovery by pigment epithelium-derived factor secretion in a rat model of cavernous nerve injury. Stem Cells Int. 2016; 2016:5161248. DOI:
10.1155/2016/5161248. PMID:
26783403. PMCID:
PMC4691496.

31. Lin H, Dhanani N, Tseng H, Souza GR, Wang G, Cao Y, Ko TC, Jiang H, Wang R. Nanoparticle improved stem cell therapy for erectile dysfunction in a rat model of cavernous nerve injury. J Urol. 2016; 195:788–795. DOI:
10.1016/j.juro.2015.10.129. PMID:
26519654.

32. Wu H, Tang WH, Zhao LM, Liu DF, Yang YZ, Zhang HT, Zhang Z, Hong K, Lin HC, Jiang H. Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury: in vivo cell tracking, optimized injection dosage, and functional evaluation. Asian J Androl. 2018; 20:442–447. DOI:
10.4103/aja.aja_48_18. PMID:
30004040. PMCID:
PMC6116694.

33. Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010; 7:3331–3340. DOI:
10.1111/j.1743-6109.2010.01875.x. PMID:
20561166. PMCID:
PMC3885341.

34. Lin G, Albersen M, Harraz AM, Fandel TM, Garcia M, McGrath MH, Konety BR, Lue TF, Lin CS. Cavernous nerve repair with allogenic adipose matrix and autologous adipose-derived stem cells. Urology. 2011; 77:1509.e1501–e1508. DOI:
10.1016/j.urology.2010.12.076. PMID:
21492917. PMCID:
PMC3158137.

35. You D, Jang MJ, Kim BH, Song G, Lee C, Suh N, Jeong IG, Ahn TY, Kim CS. Comparative study of autologous stromal vascular fraction and adipose-derived stem cells for erectile function recovery in a rat model of cavernous nerve injury. Stem Cells Transl Med. 2015; 4:351–358. DOI:
10.5966/sctm.2014-0161. PMID:
25792486. PMCID:
PMC4367505.

36. Bae JH, Shrestha KR, Park YH, Kim IG, Piao S, Jung AR, Jeon SH, Park KD, Lee JY. Comparison between subcutaneous injection of basic fibroblast growth factor-hydrogel and intracavernous injection of adipose-derived stem cells in a rat model of cavernous nerve injury. Urology. 2014; 84:1248.e1241–e1247. DOI:
10.1016/j.urology.2014.07.028. PMID:
25443945.

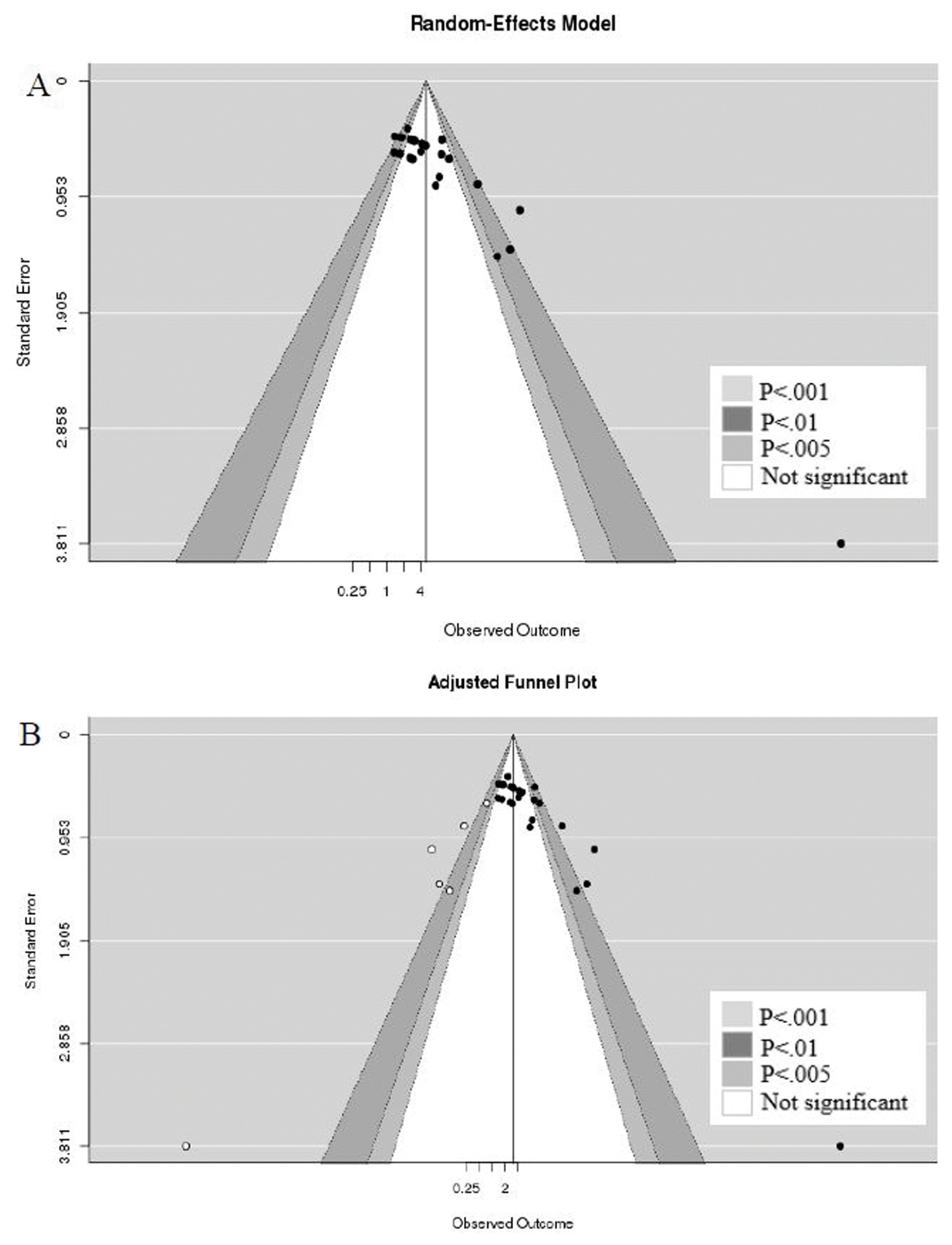
37. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56:455–463. DOI:
10.1111/j.0006-341X.2000.00455.x. PMID:
10877304.

38. Hallén K, Gustafsson LE, Wiklund NP. Nerve-induced release of nitric oxide from the rabbit corpus cavernosum is modulated by cyclic GMP. Faseb J. 2005; 19:A1205. DOI:
10.1016/j.neuroscience.2005.02.015. PMID:
15893640.

39. Jeong H, Yim HW, Cho Y, Park HJ, Jeong S, Kim HB, Hong W, Kim H. The effect of rigorous study design in the research of autologous bone marrow-derived mononuclear cell transfer in patients with acute myocardial infarction. Stem Cell Res Ther. 2013; 4:82. DOI:
10.1186/scrt233. PMID:
23849537. PMCID:
PMC3854784.

40. Conradi U, Joffe AR. Publication bias in animal research presented at the 2008 Society of Critical Care Medicine Conference. BMC Res Notes. 2017; 10:262. DOI:
10.1186/s13104-017-2574-0. PMID:
28683761. PMCID:
PMC5501347.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download