The effects of TGF-
β1 are dependent on a diversity of several parameters such as culture media, pretreatment, incubation time, cell type and differentiation (
25). One of these differences is also shown in this study by transmission into MSCs of TGF-
β1 with gene transfer. The aim of this study was to investigate the effect of TGF-
β1 gene therapy on the surface markers, multi lineage differentiation, viability, apoptosis, cell cycle, DNA damage and senescence of hDPSC. On the other hand, according to our study results, we think that TGF-
β1 overexpression with gene transfer may improve the biological potentials of the MSCs and can be a option instead of transmission of recombinant protein into cell from outside. Our results showed that TGF-
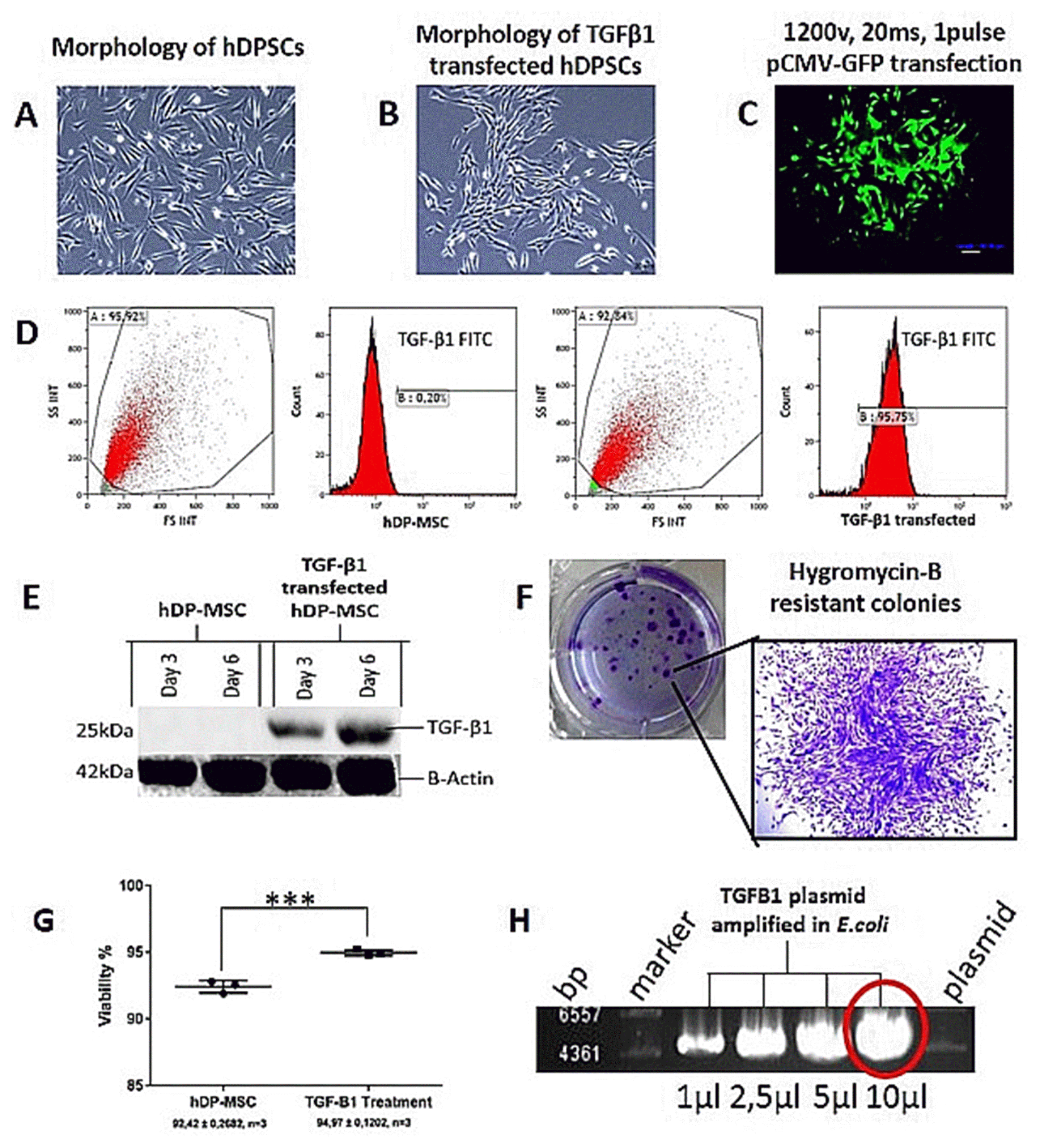
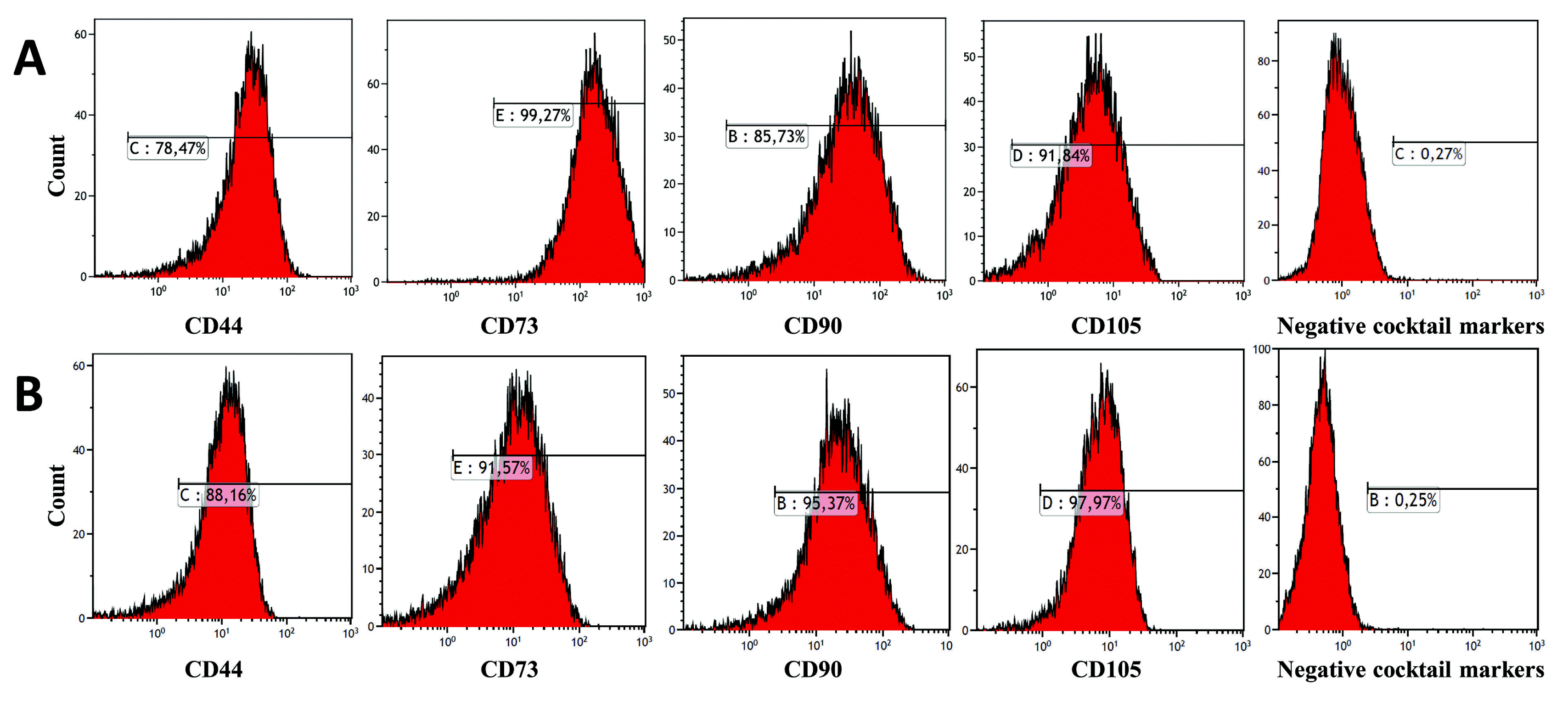
β1 has no impact on MSC immunophenotype; however, there was increased growth and differentiation; and there was no evidence that it induces cellular senescence and apoptosis. The MSCs were compared on the basis of their morphological properties, proliferation rates, expression of common MSC markers such as positive CD90, CD105, CD44, CD73 and negative CD34, CD45 and
in vitro differentiation potentials into adipocytes, osteoblasts, and chondrocytes (
26). A combination of TGF-
β1, PDGF, and
β-FGF was sufficient to grow MSCs in a serum-free medium up to 5 passages.
β-FGF, TGF-
β, and PDGF signaling is also important for MSC growth (
17). Our results showed that the continuous TGF-
β1 overexpression in hDPSCs did not negatively influence the immunophenotype and surface marker expression of MSCs. The TGF-
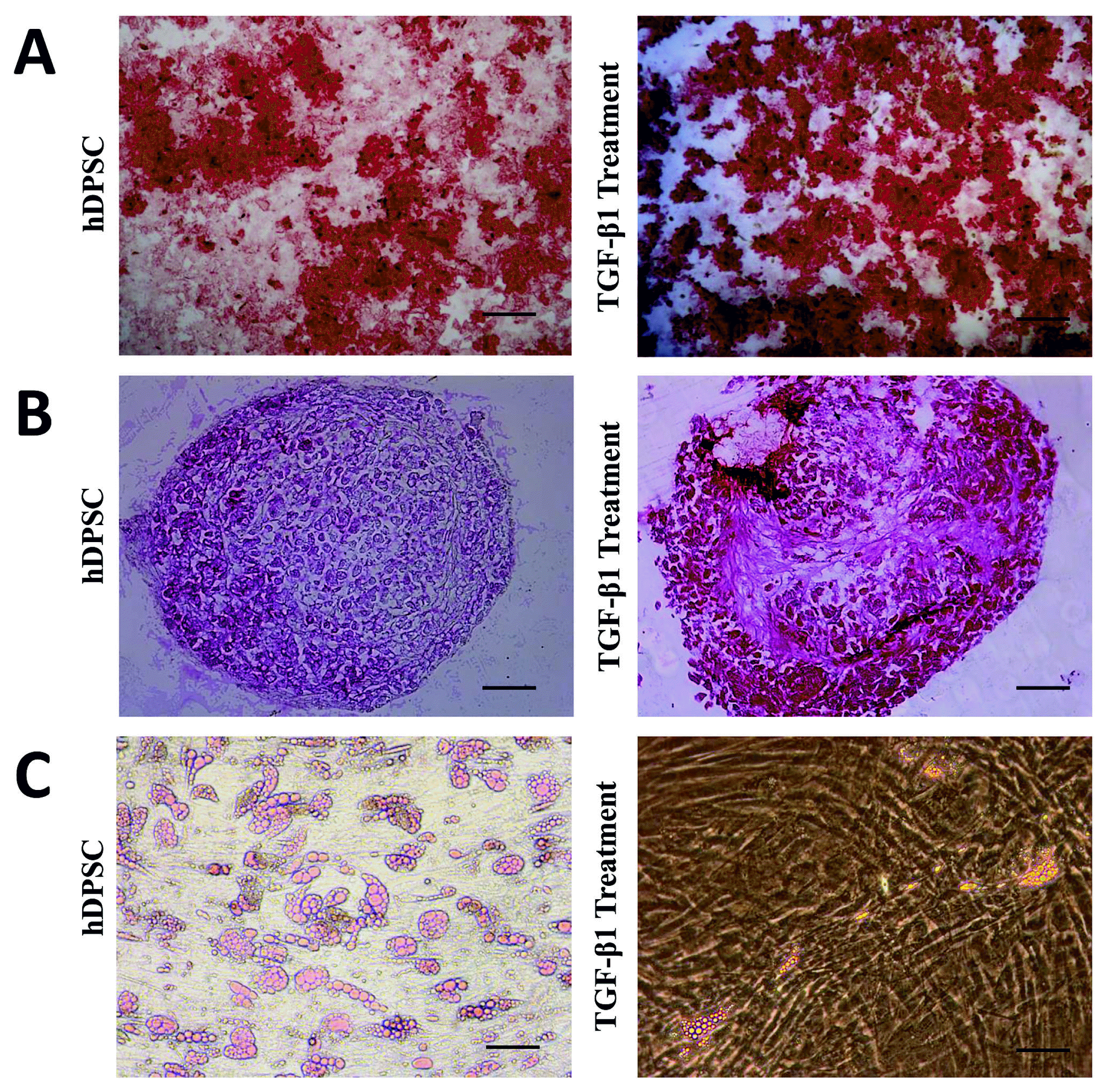
β1 signal pathway was shown to have a critical role in the osteogenic and chondrogenic differentiation of MSCs. TGF-
β1 is also an inhibitor of adipogenesis via SMAD 3 signaling. Our results support these data reported in the literature on TGFB1, because TGF-
β1 increased osteogenic and chondrogenic differentiation but decreased adipogenic differentiation. The activin/nodal pathway, which signals through the TGF-
β1 pathway, cooperates with FGF signaling in maintaining the pluripotency of embryonic stem cells. We found in this study that overexpression of TGF-
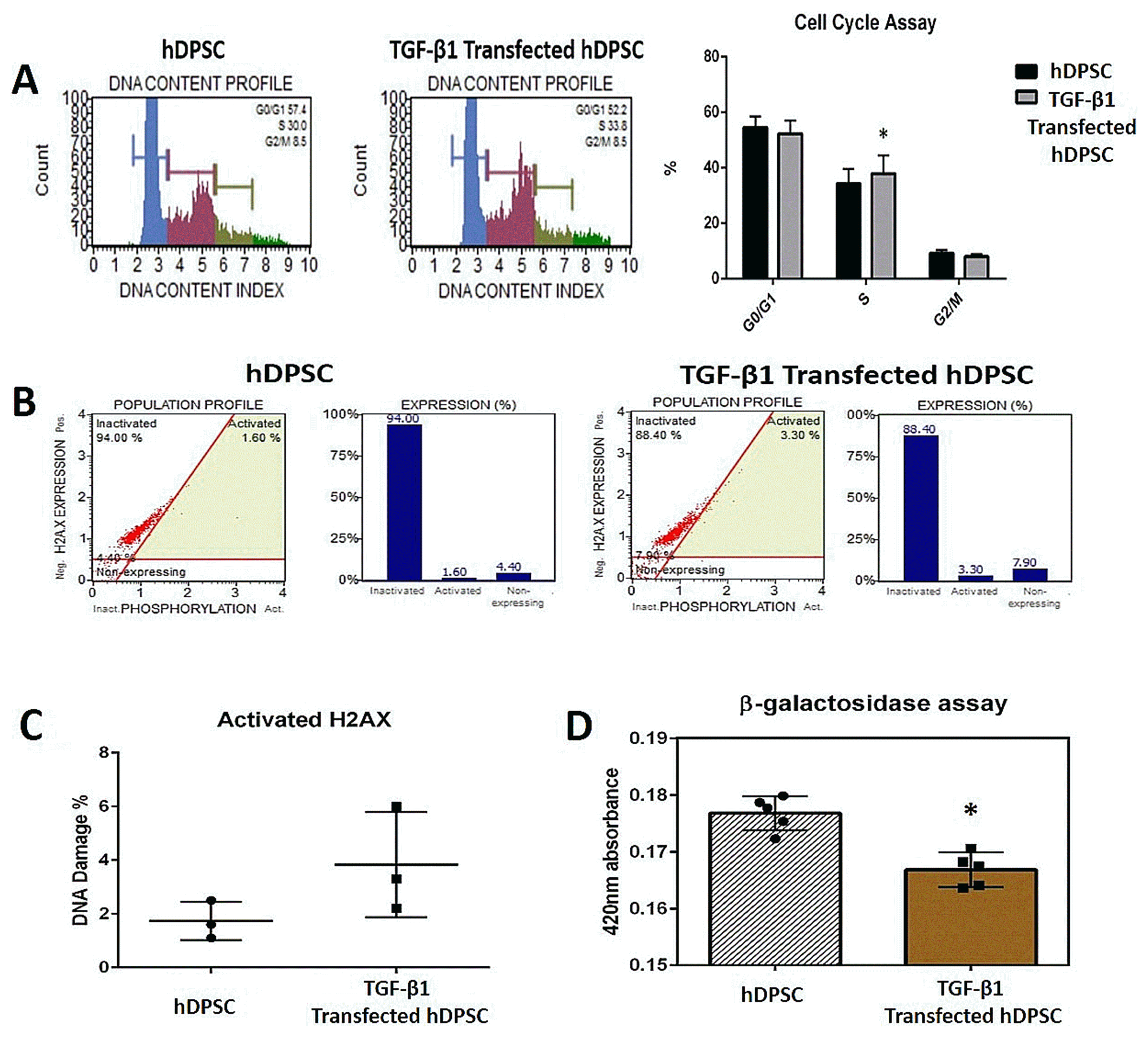
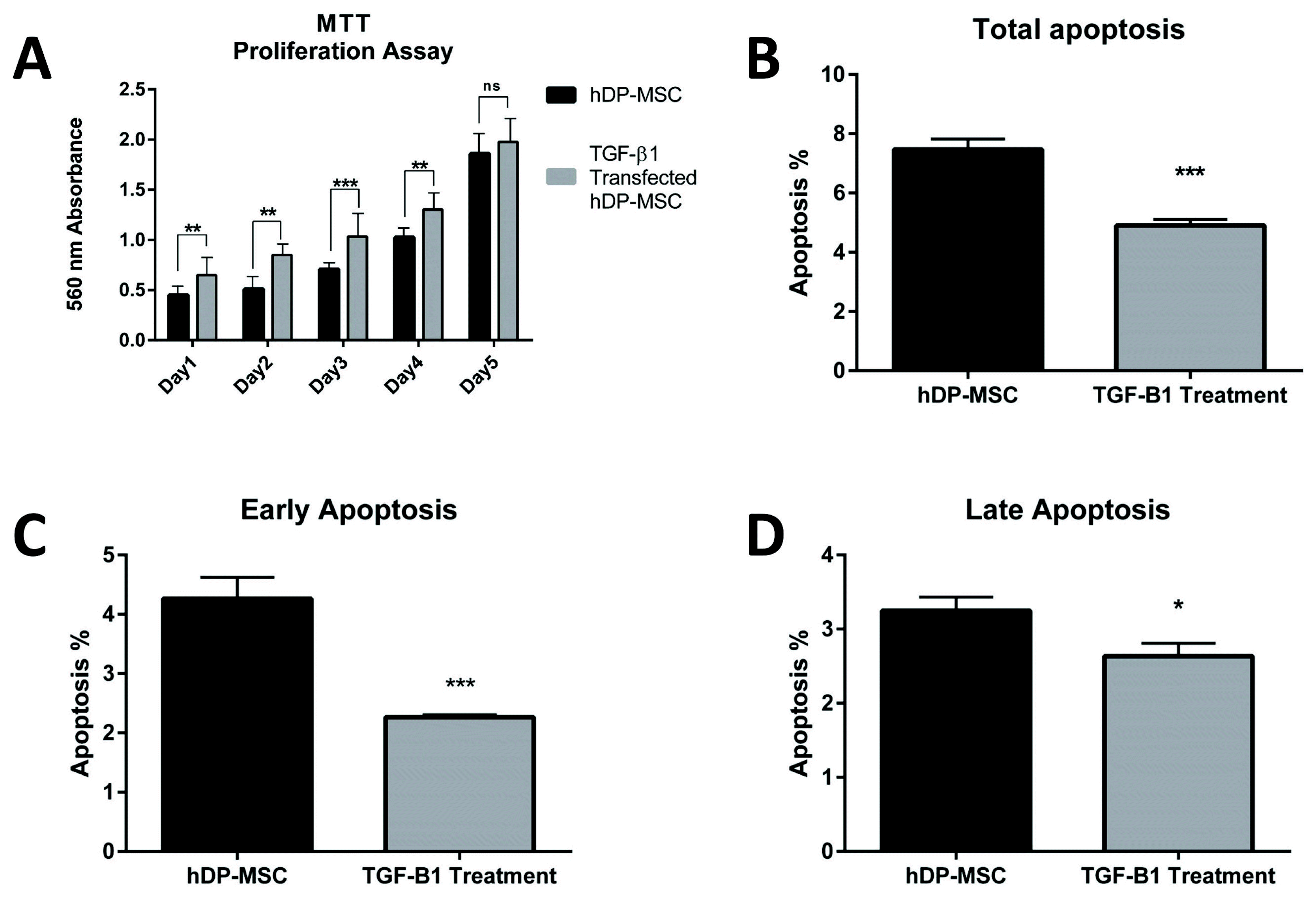
β1 with gene transfer had higher proliferation rate and decreased total apoptosis in hDPSCs (p<0.05); and the number of cells at “
S” phase was higher with TGF-
β1 transfection. However, there was no significant difference at DNA damage. Jian H et al. (2006) showed that TGF-
β1 revealed unique biological effects in specific cellular condition of human MSCs, as it induces the proliferation of those cells, which is in contrast to the strong anti-proliferative effect of TGF-
β1 on many other cell types, suggesting that TGF-
β1 may induce particular biological responses through the integrated actions of
β-catenin and SMAD proteins in the nucleus to regulate the expression of a specific set of TGF-
β1 target genes. TGF-
β single or in a mix with Platelet-Derived Growth Factor (PDGF) and Fibroblast Growth Factor (FGF) was suggested to be required to enable
in vitro proliferation of MSCs (
17–
27), whereas other studies demonstrated that it induces cell-cycle arrest in mesodermal cells (
28,
29). Some of these conflicting results may be due to the heterogeneous composition of different MSC isolation methods or culture requirement (
30). In our study, we found that cellular senescence decreased in TGF-
β1 transfected group. Senescence result in a permanent cell cycle arrest and MSCs lose their self-renewal potential (
21). The superior proliferative ability and regenerative potential are major phenotypes of MSCs (
22). Loss of regenerative potential of MSCs would limit their practice in regenerative therapy. TGF-
β1 has been reported to induce senescence in tumor cells and other cells (
23,
24). Wu et al. (2014) studied the effect of TGF-
β1 on senescence of BMMSCs. Their conclusion showed that treatment with TGF-
β1 (1~10 ng/mL) increased SA-Gal activity and mtROS production in BMSCs in a dose-dependent form (
31). However, Walenda et al. (2013) reported that TGF-
β1 did not stimulate early senescence. This was also evident, when they analyzed senescence-associated DNA methylation modifies. They suggested that gene expression profiles of MSCs differed remarkably at relatively early (P 3~5) and later passages (P 10). Nonetheless, they also reported that relative gene expression variations provoked by TGF-
β1 in a time course-dependent manner were very parallel in MSCs of early and late passage. These results support the idea that TGF-
β1 has great impact on MSC function and has similar molecular effects during culture expansion (
25). It has been reported in previous transfection studies in the literature that transfected cells preserve the transfer efficiency even after long-term culture, and the transferred gene is expressed in a continuous manner after the gene transfer. Kim et al. (2014) studied the effect of TGF-
β1 overexpression on the chondrogenic differentiation of synovial stem cells; and the TGF-
β1 gene product was transferred into synovial stem cells through retroviral vectors. Even on day 15 after the transfer, it was shown with the ELISA test that the TGF-
β1 in the conditioned medium of the transfected cells were at high levels; and TGF-
β1 protein levels increased gradually depending on time in the culture medium. To compare the MSC surface marker expressions in TGF-
β1 transfected and non-transfected synovial fluid stem cells, long-term culture was carried out in the transfected cells for 21 days, and it was determined that the surface marker expression of the cells was not affected even on the 21
st day (
32). These data support the results we obtained in the present study. In our study, it was shown by us that the MSC surface marker expressions of the TGF-
β1 transfected hDPSCs in the 3
rd passage (approximately on the 20~21
st days of the culture) were not affected in post-transfection cultures. The researchers showed that after the transfection with retroviral TGF-
β1 transfected cells, the transfected cells formed aggregates by culturing with chondrogenic medium; and even that these aggregates were larger, and differentiated more chondrogenically in the transfected cell groups (
32). In our study, it was determined that the TGF-
β1 transfected cells were cultured in 3 dimensional cultures for 21 days with chondrogenic induction to form aggregates; and the TGF-
β1 gene transfer increased chondrogenic differentiation in hDPSCs. In a study reporting that TGF-
β1 overexpression triggered homing in BMMSCs in renal ischemia-reperfusion injury, the TGF-
β1 plasmid was transfected into BMMSCs with lentiviral vectors; and the Fluorescent Method was used to determine the transfection efficacy 48 hours after the transfection (
33). In our study, the TGF-
β1 plasmid was transferred to hDPSCs with electroporation; and the transfection efficacy was measured with flow cytometry at the 48
th hour as 95%. It was determined with western blot analysis that the TGF-
β1 protein expression increased at a significant level on the 3
rd and 6
th days compared to non-transfected DPSCs; and the measurements of western blot analysis were supported by the flow cytometry. In addition, the transfection success was shown by us under fluorescence microscope with the transfer of the GFP plasmid to the cells. These reports in the literature about the stability of the gene that is transferred and the culture conditions after the transfection support the results of our study. It was also shown in our study with flow cytometry, western blot, CFU-F, hygromycin resistance, and surface marker expression multilineage differentiation tests that long-term gene transfer stability is obtained in cells. In a study in which transfection parameters were optimized with electroporation in DPSCs the transfection efficiency was measured with the flow cytometry and western blot analysis at the 48
th and 72
nd hours (
10). The findings we mentioned in the study showing that the MSC surface marker expressions were not affected after the transfection were supported in the study conducted by Rizk et al. by showing that CD44 was positively expressed in TGF-
β3 transfected cells and CD45 was negatively expressed (
10). These results show that TGF-
β transfection affect the MSC surface markers. This situation shows that we produced cells, which can better differentiate without impairing the immunophenotype, which affect their biological characteristics better, and which have better usage and yield potential in terms of regenerative medicine. In our study, there is hygromycin b resistance gene area as the eukaryotic selective marker in the plasmid which was transfected. The TGF-
β1 transfected cells were used to guarantee the permanent integration of the transferred gene (to which hygromycin b antibiotic was transferred) to the chromosome in the complete medium at 50
μg/ml in the culture medium; and the experiments were established with the hDPSC, which received the TGF-
β1 gene permanently. Liu et al. conducted a study and also reported that the long-term culture after transfection did not affect the cells negatively, and the stability of the transferred gene was ensured. The researchers transferred the Brain-Derived Neurotrophic Factor Gene (BDNF) to the cells with transfection in the differentiation of bone marrow-derived mesenchymal stem cells into nerve-like cells. Since the transferred plasmid geneticin (G418) has a selective marker, the cells were selected for 14 days with selective antibiotics as in our experiment plan. The ELISA test results showed that the BDNF gene product that was transferred was at high levels even after 2 months in cell supernatants (
34). The long-term culture conditions of the transfected cells show that they do not affect them negatively, which was also the case in our study. It was reported by Kim et al. that TGF-
β1 transfection not only increased the chondrogenesis but also increased the proliferation in MSCs (
32). In our study, the TGF-
β1 transfection increased the proliferation in hDPSCs at a significant level. Despite these studies, which we mentioned as being associated with TGF-
β1 transfection in the literature, there are no comprehensive studies conducted on how the TGF-
β1 transfection affects the MSCs cell characteristics. The existing studies remain at proliferation and multilineage differentiation level. Moreover, the variables such as cell cycle, DNA damage and cellular senescence of the Dental Pulp Mesenchymal Stromal Cells after TGF-
β1 overexpression were investigated in our study. The present study of ours showed that TGF-
β1 overexpression affect Dental Pulp Mesenchymal Stromal Cells in a positive way. These results reflect that TGF-
β1 has major impact on MSC differentiation. TGF-
β1 transfection has no effect on cell surface markers. TGF-
β1 transfection has positive effects on proliferation, cell cycle and prevents cellular senescence and apoptosis (
Table 1). In further studies, it will be essential to determine whether TGF-
β1 can play a role in attempts to use MSC for therapeutic approaches. With this study, cells were produced with increased differentiation potentials and strengthened biological features that may be used in regenerative medicine, tissue engineering, gene therapy and cellular therapy studies.
Table 1
This table shown that results statistically of viability, apoptosis, Cell cycle, DNA damage and senescence assay
|
Experiments |
Avarege±SD (hDPSCs) |
Avarege±SD (TGFB1 transfected hDPSCs) |
Number of values |
p-value |
|
Viability |
92.42±0.2682 |
94.97±0.1202 |
3 |
0.0005 |
|
Early Apoptosis |
4.267±0.2048 |
2.267±0.01667 |
3 |
0.0006 |
|
Late Apoptosis |
3.250±0.1041 |
2.633±0.1014 |
3 |
0.0132 |
|
Total Apoptosis |
7.467±0.2048 |
4.900±0.1155 |
3 |
0.0004 |
|
Cell Cycle (S phase) |
31.27+0.8192 |
34.70+0.6658 |
3 |
0.0313 |
|
DNA Damage |
1.733±0.4096 |
2.733±0.2963 |
3 |
0.1191 |
|
Senescence |
0.1768±0.001345 |
0.1668±0.001363 |
5 |
0.0008 |





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download