1. Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001; 268:189–200. DOI:
10.1006/excr.2001.5278. PMID:
11478845.

2. Ichinose S, Tagami M, Muneta T, Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res. 2005; 322:217–226. DOI:
10.1007/s00441-005-1140-6. PMID:
16091918.

3. Nöth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002; 8:131–144. DOI:
10.1089/107632702753503126. PMID:
11886661.

4. Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002; 99:4397–4402. DOI:
10.1073/pnas.052716199. PMID:
11917104. PMCID:
123659.

5. Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004; 6:596–601. DOI:
10.1080/14653240410005276-1.

6. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998; 238:265–272. DOI:
10.1006/excr.1997.3858. PMID:
9457080.

7. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998; 4:415–428. DOI:
10.1089/ten.1998.4.415. PMID:
9916173.

8. Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003; 48:418–429. DOI:
10.1002/art.10767. PMID:
12571852.

9. Mwale F, Girard-Lauriault PL, Wang HT, Lerouge S, Antoniou J, Wertheimer MR. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006; 12:2639–2647. DOI:
10.1089/ten.2006.12.2639. PMID:
16995797.

10. Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008; 58:1377–1388. DOI:
10.1002/art.23370. PMID:
18438858. PMCID:
3612425.

11. Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P. Hypertrophy in mesenchymal stem cell chondrogenesis: effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs. 2010; 192:158–166. DOI:
10.1159/000313399. PMID:
20407224. PMCID:
2968769.

12. Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006; 54:3254–3266. DOI:
10.1002/art.22136. PMID:
17009260.

13. Scotti C, Tonnarelli B, Papadimitropoulos A, Scherberich A, Schaeren S, Schauerte A, Lopez-Rios J, Zeller R, Barbero A, Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010; 107:7251–7256. DOI:
10.1073/pnas.1000302107. PMID:
20406908. PMCID:
2867676.

14. Seyedin SM, Thompson AY, Bentz H, Rosen DM, McPherson JM, Conti A, Siegel NR, Galluppi GR, Piez KA. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986; 261:5693–5695. PMID:
3754555.

15. Schofield JN, Wolpert L. Effect of TGF-beta 1, TGF-beta 2, and bFGF on chick cartilage and muscle cell differentiation. Exp Cell Res. 1990; 191:144–148. DOI:
10.1016/0014-4827(90)90048-F. PMID:
2226646.

16. Leonard CM, Fuld HM, Frenz DA, Downie SA, Massagué J, Newman SA. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev Biol. 1991; 145:99–109. DOI:
10.1016/0012-1606(91)90216-P. PMID:
2019328.

17. Joyce ME, Roberts AB, Sporn MB, Bolander ME. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990; 110:2195–2207. DOI:
10.1083/jcb.110.6.2195. PMID:
2351696. PMCID:
2116133.

18. Chimal-Monroy J, Díaz de León L. Differential effects of transforming growth factors beta 1, beta 2, beta 3 and beta 5 on chondrogenesis in mouse limb bud mesenchymal cells. Int J Dev Biol. 1997; 41:91–102. PMID:
9074941.
19. Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993; 158:414–429. DOI:
10.1006/dbio.1993.1200. PMID:
8344460.

20. Böhme K, Winterhalter KH, Bruckner P. Terminal differentiation of chondrocytes in culture is a spontaneous process and is arrested by transforming growth factor-beta 2 and basic fibroblast growth factor in synergy. Exp Cell Res. 1995; 216:191–198. DOI:
10.1006/excr.1995.1024. PMID:
7813620.

21. Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000; 141:4728–4735. DOI:
10.1210/endo.141.12.7848. PMID:
11108288.

22. Ferguson CM, Schwarz EM, Puzas JE, Zuscik MJ, Drissi H, O’Keefe RJ. Transforming growth factor-beta1 induced alteration of skeletal morphogenesis in vivo. J Orthop Res. 2004; 22:687–696. DOI:
10.1016/j.orthres.2003.10.023. PMID:
15183422.

23. Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997; 139:541–552. DOI:
10.1083/jcb.139.2.541. PMID:
9334355. PMCID:
2139797.

24. Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. J Cell Biol. 2001; 153:35–46. DOI:
10.1083/jcb.153.1.35. PMID:
11285272. PMCID:
2185521.

25. Matsunobu T, Torigoe K, Ishikawa M, de Vega S, Kulkarni AB, Iwamoto Y, Yamada Y. Critical roles of the TGF-beta type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Dev Biol. 2009; 332:325–338. DOI:
10.1016/j.ydbio.2009.06.002. PMID:
19501582. PMCID:
2716725.

26. Cooley JR, Yatskievych TA, Antin PB. Embryonic expression of the transforming growth factor beta ligand and receptor genes in chicken. Dev Dyn. 2014; 243:497–508. DOI:
10.1002/dvdy.24085. PMID:
24166734. PMCID:
3969743.

27. Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007; 177:1105–1117. DOI:
10.1083/jcb.200611031. PMID:
17576802. PMCID:
2064369.
28. Hayata T, Ezura Y, Asashima M, Nishinakamura R, Noda M. Dullard/Ctdnep1 regulates endochondral ossification via suppression of TGF-β signaling. J Bone Miner Res. 2015; 30:318–329. DOI:
10.1002/jbmr.2343. PMID:
25155999.

29. Matsunaga S, Yamamoto T, Fukumura K. Temporal and spatial expressions of transforming growth factor-betas and their receptors in epiphyseal growth plate. Int J Oncol. 1999; 14:1063–1067. PMID:
10339658.

30. Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, Lefebvre V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012; 22:597–609. DOI:
10.1016/j.devcel.2011.12.024. PMID:
22421045. PMCID:
3306603.

31. Yamashita S, Andoh M, Ueno-Kudoh H, Sato T, Miyaki S, Asahara H. Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp Cell Res. 2009; 315:2231–2240. DOI:
10.1016/j.yexcr.2009.03.008. PMID:
19306868. PMCID:
2696577.

32. Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002; 62:65–74. DOI:
10.1124/mol.62.1.65. PMID:
12065756.

33. Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, Heer J, Kwon C, Lehr R, Mathur A, Olson BA, Weinstock J, Laping NJ. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5). J Med Chem. 2002; 45:999–1001. DOI:
10.1021/jm010493y. PMID:
11855979.

34. Karl A, Olbrich N, Pfeifer C, Berner A, Zellner J, Kujat R, Angele P, Nerlich M, Mueller MB. Thyroid hormone-induced hypertrophy in mesenchymal stem cell chondrogenesis is mediated by bone morphogenetic protein-4. Tissue Eng Part A. 2014; 20:178–188. DOI:
10.1089/ten.tea.2013.0023. PMID:
23937304. PMCID:
3875213.

35. Kawamura I, Maeda S, Imamura K, Setoguchi T, Yokouchi M, Ishidou Y, Komiya S. SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-β and bone morphogenetic protein pathways. J Biol Chem. 2012; 287:29101–29113. DOI:
10.1074/jbc.M112.349415. PMID:
22767605. PMCID:
3436559.

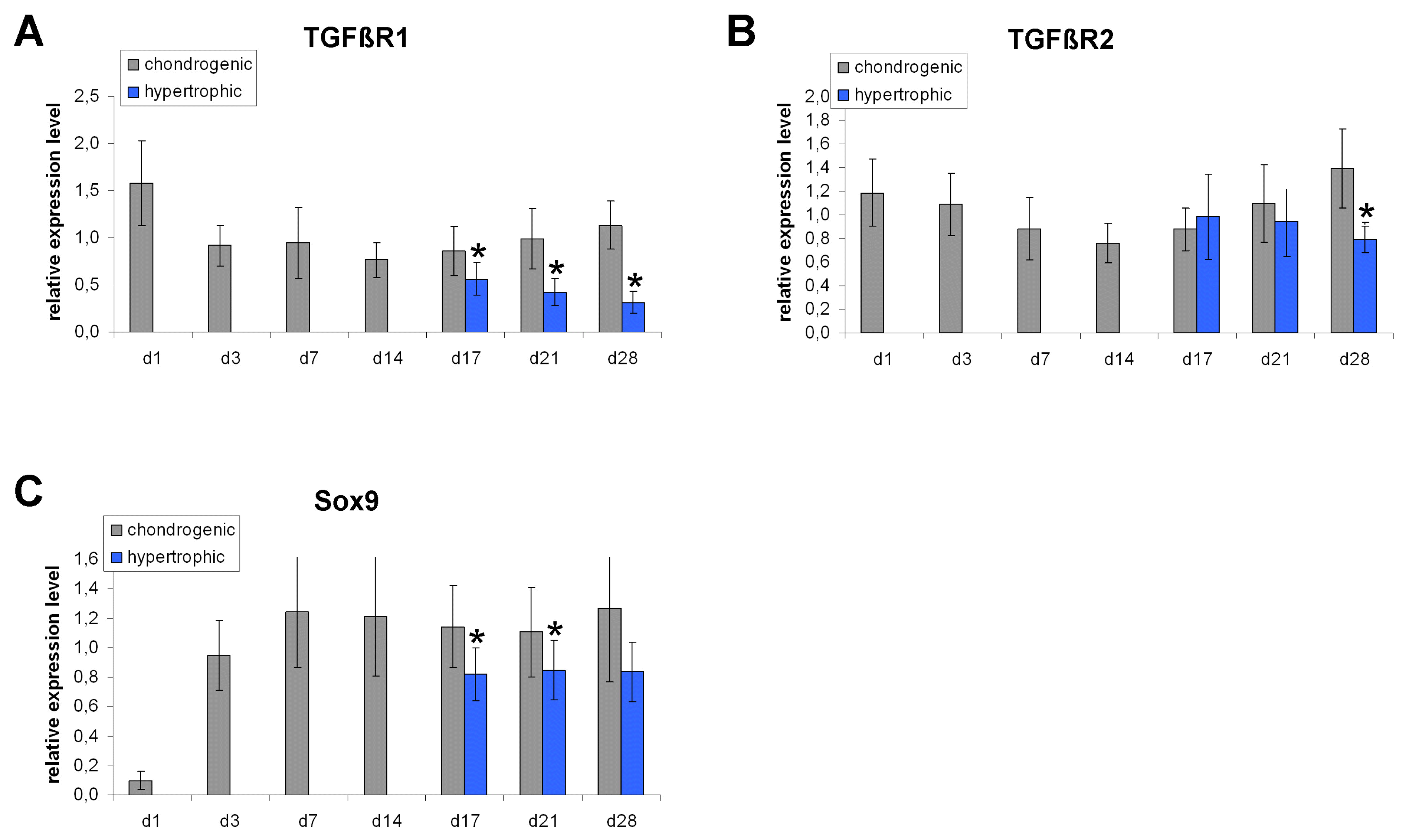
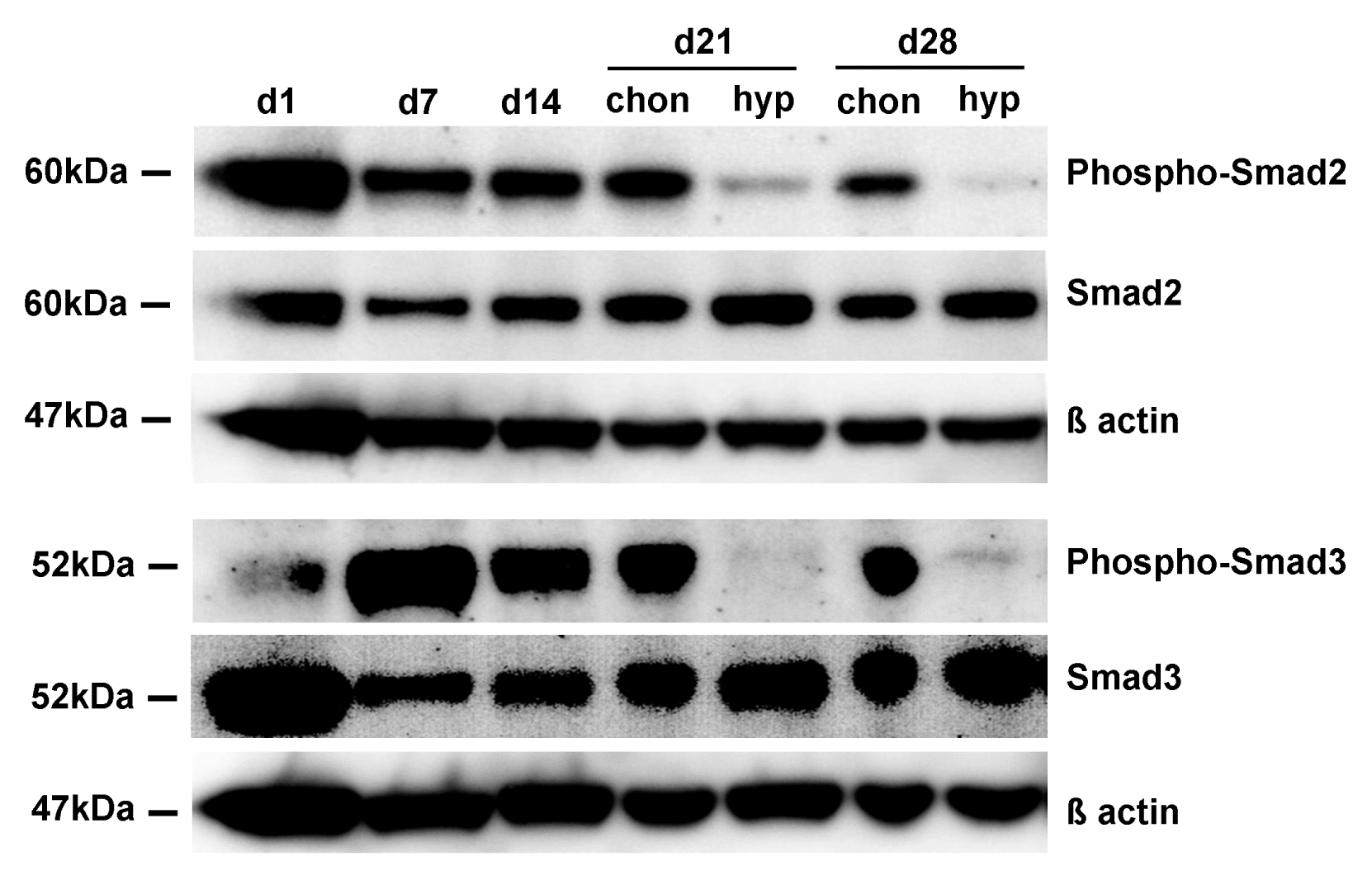





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download