2. Castellani R, Smith MA, Richey PL, Perry G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996; 737:195–200. DOI:
10.1016/0006-8993(96)00729-9. PMID:
8930366.

3. Münch G, Lüth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P. Crosslinking of alpha-synuclein by advanced glycation endproducts--an early pathophysiological step in Lewy body formation? J Chem Neuroanat. 2000; 20:253–257. DOI:
10.1016/S0891-0618(00)00096-X.

4. Cepeda IL, Flores J, Cornfeldt ML, O’Kusky JR, Doudet DJ. Human retinal pigment epithelial cell implants ameliorate motor deficits in two rat models of Parkinson disease. J Neuropathol Exp Neurol. 2007; 66:576–584. DOI:
10.1097/nen.0b013e318093e521. PMID:
17620983.

7. Fu MH, Li CL, Lin HL, Chen PC, Calkins MJ, Chang YF, Cheng PH, Yang SH. Stem cell transplantation therapy in Parkinson’s disease. Springerplus. 2015; 4:597. DOI:
10.1186/s40064-015-1400-1. PMID:
26543732. PMCID:
4628010.

8. Lindvall O. Treatment of Parkinson’s disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140370. DOI:
10.1098/rstb.2014.0370. PMID:
26416681. PMCID:
4633999.

9. Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Mov Disord. 2010; 25(Suppl 1):S55–57. DOI:
10.1002/mds.22638. PMID:
20187228.

10. Damien P, Allan DS. Regenerative therapy and immune modulation using umbilical cord blood-derived cells. Biol Blood Marrow Transplant. 2015; 21:1545–1554. DOI:
10.1016/j.bbmt.2015.05.022. PMID:
26079441.

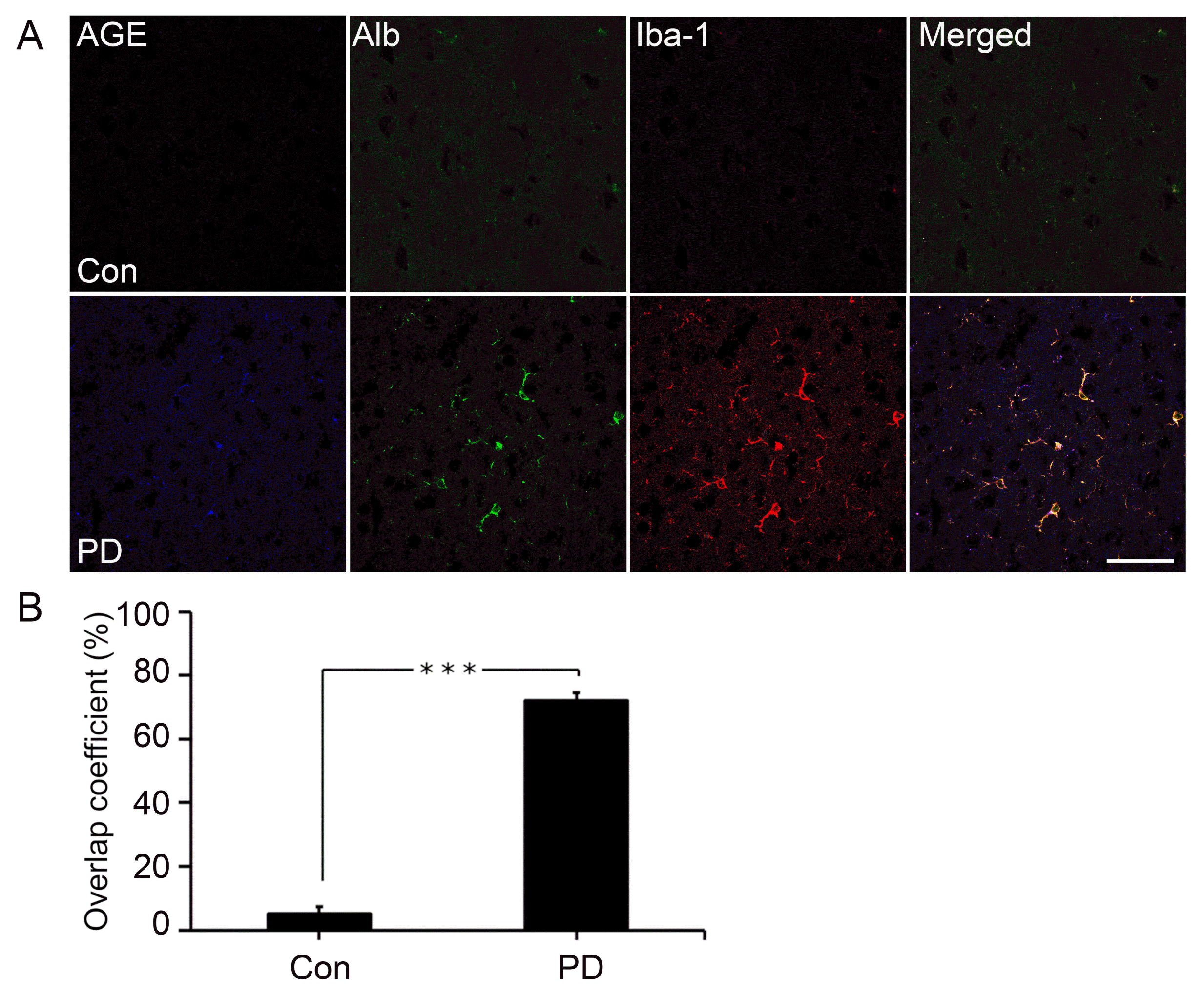
11. Bayarsaikhan E, Bayarsaikhan D, Lee J, Son M, Oh S, Moon J, Park HJ2, Roshini A2, Kim SU4, Song BJ5, Jo SM6, Byun K3, Lee B3. Microglial AGE-albumin is critical for neuronal death in Parkinson’s disease: a possible implication for theranostics. Int J Nanomedicine. 2016; 10(Spec Iss):281–292. DOI:
10.2147/IJN.S95077. PMID:
27601894. PMCID:
5003553.
12. Kalea AZ, Schmidt AM, Hudson BI. Alternative splicing of RAGE: roles in biology and disease. Front Biosci (Landmark Ed). 2011; 16:2756–2770. DOI:
10.2741/3884.

13. Son M, Kang WC, Oh S, Bayarsaikhan D, Ahn H, Lee J, Park H, Lee S, Choi J, Lee HS, Yang PC, Byun K, Lee B. Advanced glycation end-product (AGE)-albumin from activated macrophage is critical in human mesenchymal stem cells survival and post-ischemic reperfusion injury. Sci Rep. 2017; 7:11593. DOI:
10.1038/s41598-017-11773-1. PMID:
28912521. PMCID:
5599509.

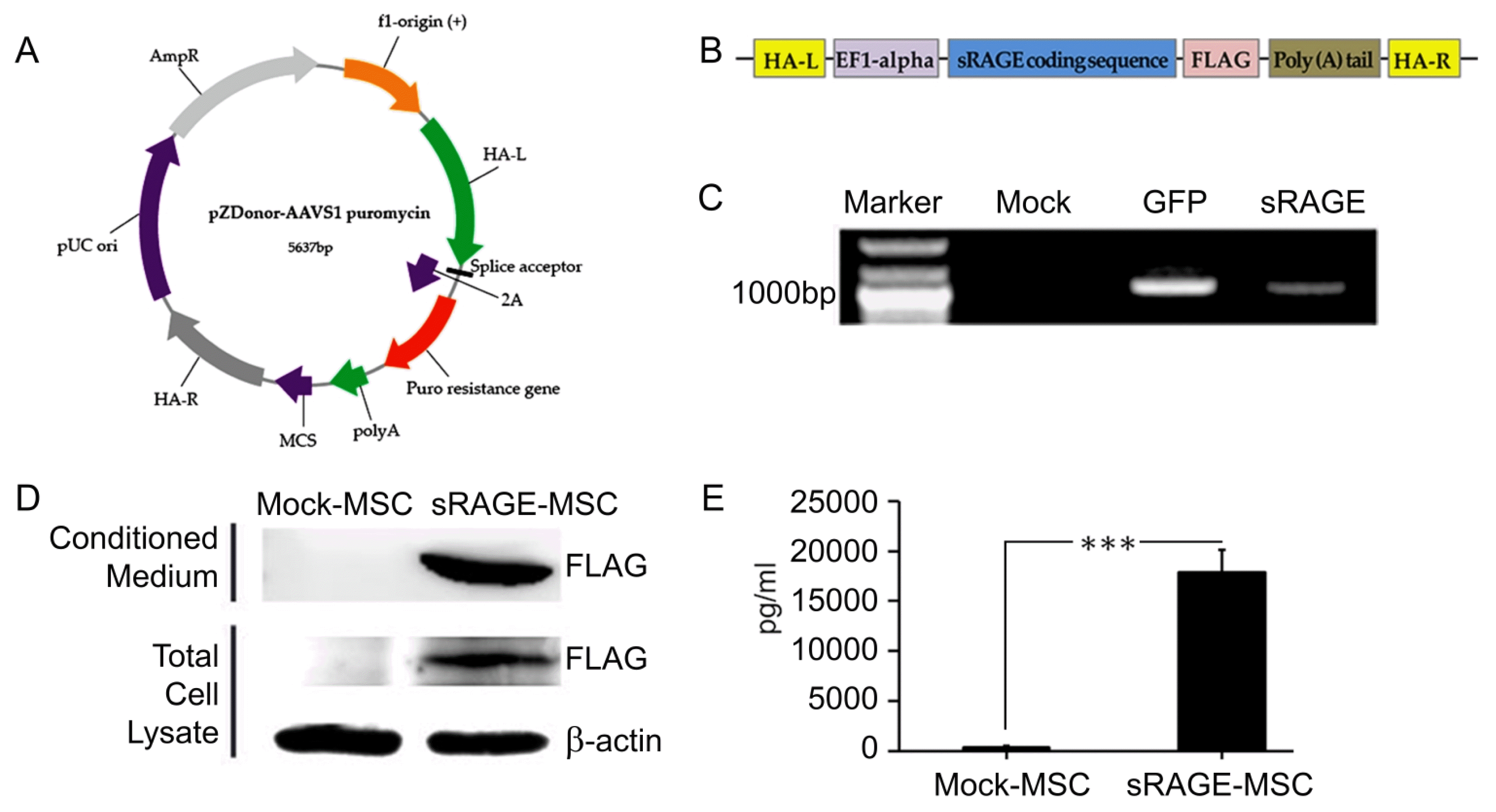
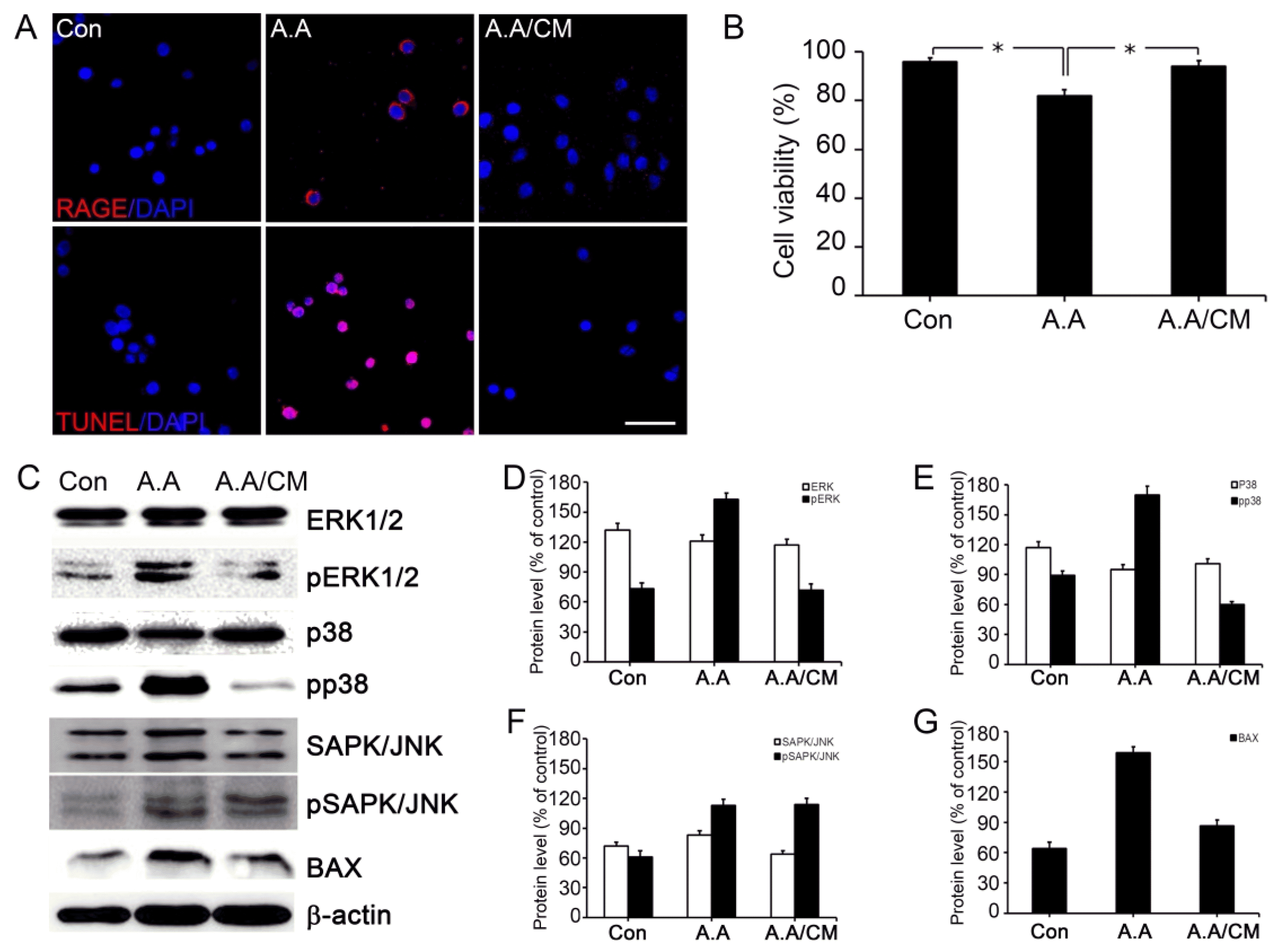
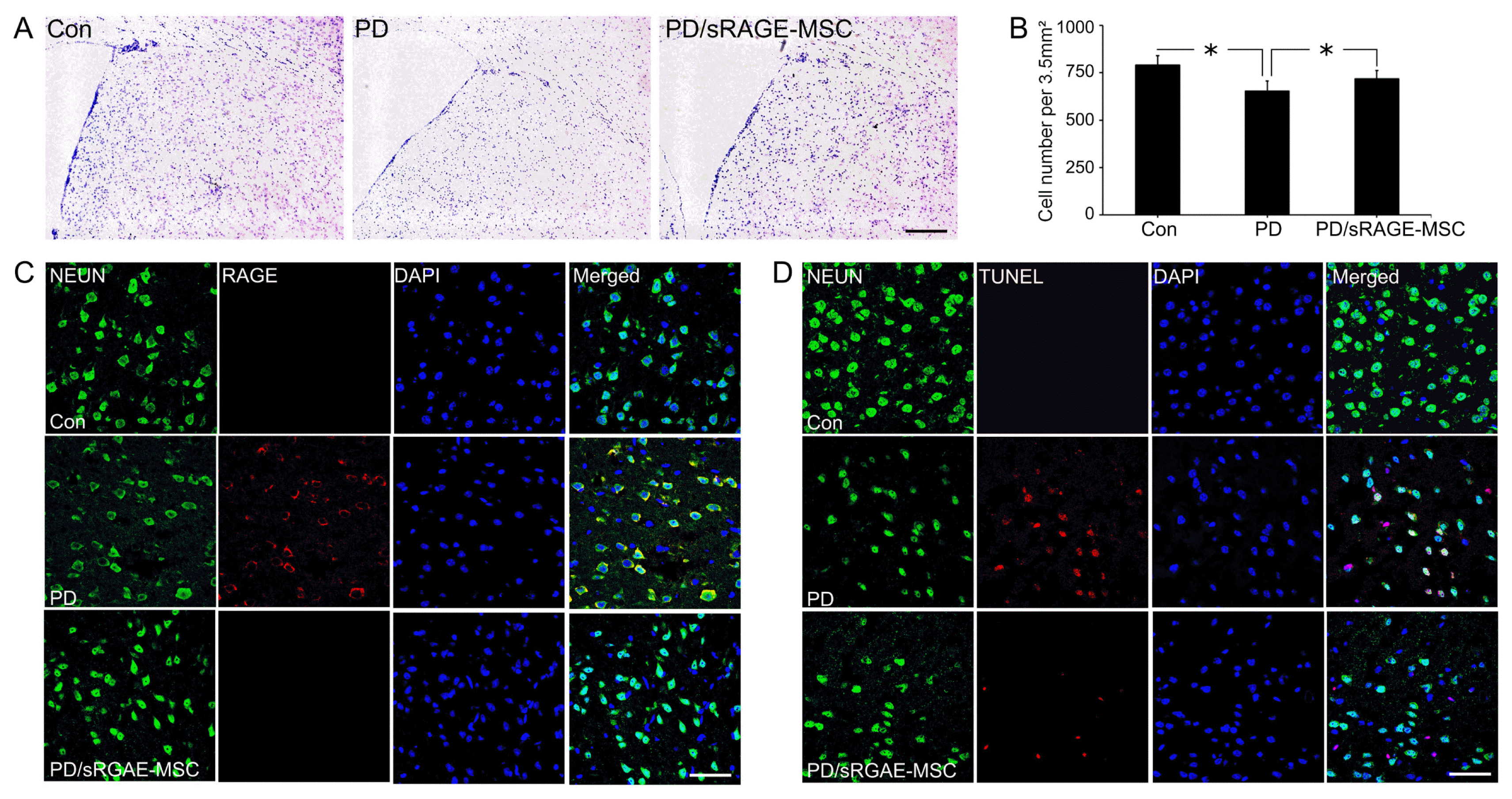
14. Son M, Oh S, Park H, Ahn H, Choi J, Kim H, Lee HS, Lee S, Park HJ, Kim SU, Lee B, Byun K. Protection against RAGE-mediated neuronal cell death by sRAGE-secreting human mesenchymal stem cells in 5xFAD transgenic mouse model. Brain Behav Immun. 2017; 66:347–358. DOI:
10.1016/j.bbi.2017.07.158. PMID:
28760504.

15. Byun K, Yoo Y, Son M, Lee J, Jeong GB, Park YM, Salekdeh GH, Lee B. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017; 177:44–55. DOI:
10.1016/j.pharmthera.2017.02.030. PMID:
28223234.

16. Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001; 108:949–955. DOI:
10.1172/JCI200114002. PMID:
11581294. PMCID:
200958.

17. Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002; 59:1117–1128. DOI:
10.1007/s00018-002-8491-x. PMID:
12222959.

18. Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, Park YM, Lee B. Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer’s disease. PLoS One. 2012; 7:e37917. DOI:
10.1371/journal.pone.0037917. PMID:
22662249. PMCID:
3360664.

19. Byun K, Bayarsaikhan E, Kim D, Son M, Hong J, Jeong GB, Paek SH, Won MH, Lee B. Activated microglial cells synthesize and secrete AGE-albumin. Anat Cell Biol. 2012; 45:47–52. DOI:
10.5115/acb.2012.45.1.47. PMID:
22536551. PMCID:
3328740.

21. Chang CY, Ting HC, Su HL, Jeng JR. Combining induced pluripotent stem cells and genome editing technologies for clinical applications. Cell Transplant. 2018; 27:379–392. DOI:
10.1177/0963689718754560. PMID:
29806481. PMCID:
6038034.

22. Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017; 168:20–36. DOI:
10.1016/j.cell.2016.10.044. PMCID:
5235943.

23. Hendriks WT, Warren CR, Cowan CA. Genome editing in human pluripotent stem cells: approaches, pitfalls, and solutions. Cell Stem Cell. 2016; 18:53–65. DOI:
10.1016/j.stem.2015.12.002. PMID:
26748756. PMCID:
4709030.

24. Ceasar SA, Rajan V, Prykhozhij SV, Berman JN, Ignacimuthu S. Insert, remove or replace: a highly advanced genome editing system using CRISPR/Cas9. Biochim Biophys Acta. 2016; 1863:2333–2344. DOI:
10.1016/j.bbamcr.2016.06.009. PMID:
27350235.

27. Chandrasegaran S. Recent advances in the use of ZFNmediated gene editing for human gene therapy. Cell Gene Ther Insights. 2017; 3:33–41. DOI:
10.18609/cgti.2017.005. PMID:
29270315. PMCID:
5736148.

28. Renaud JB, Boix C, Charpentier M, De Cian A, Cochennec J, Duvernois-Berthet E, Perrouault L, Tesson L, Edouard J, Thinard R, Cherifi Y, Menoret S, Fontanière S, de Crozé N, Fraichard A, Sohm F, Anegon I, Concordet JP, Giovannangeli C. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell Rep. 2016; 14:2263–2272. DOI:
10.1016/j.celrep.2016.02.018. PMID:
26923600.

29. Luo Y, Rao M, Zou J. Generation of GFP reporter human induced pluripotent stem cells using AAVS1 safe harbor transcription activator-like effector nuclease. Curr Protoc Stem Cell Biol. 2014; 29:5.A.7.1–18. DOI:
10.1002/9780470151808.sc05a07s29.

30. Münch G, Westcott B, Menini T, Gugliucci A. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids. 2012; 42:1221–1236. DOI:
10.1007/s00726-010-0777-y. PMID:
20949363.

32. Vasan S, Foiles PG, Founds HW. Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin Investig Drugs. 2001; 10:1977–1987. DOI:
10.1517/13543784.10.11.1977. PMID:
11772301.

33. Walker DG, Lue LF. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. J Neurosci Res. 2005; 81:412–425. DOI:
10.1002/jnr.20484. PMID:
15957156.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download