Introduction
Materials and Methods
Human mesenchymal stem cell culture
Lentiviral vector construction and virus production
Lentiviral transduction
Immunocytochemistry
Spinal cord contusion injury
Behavioral assessment after spinal cord injury
Cell transplantation
Histological processing and immunohistochemistry
Histological analysis of tissue injury
Statistical analysis
Results
Spinal cord injury and cell transplantation
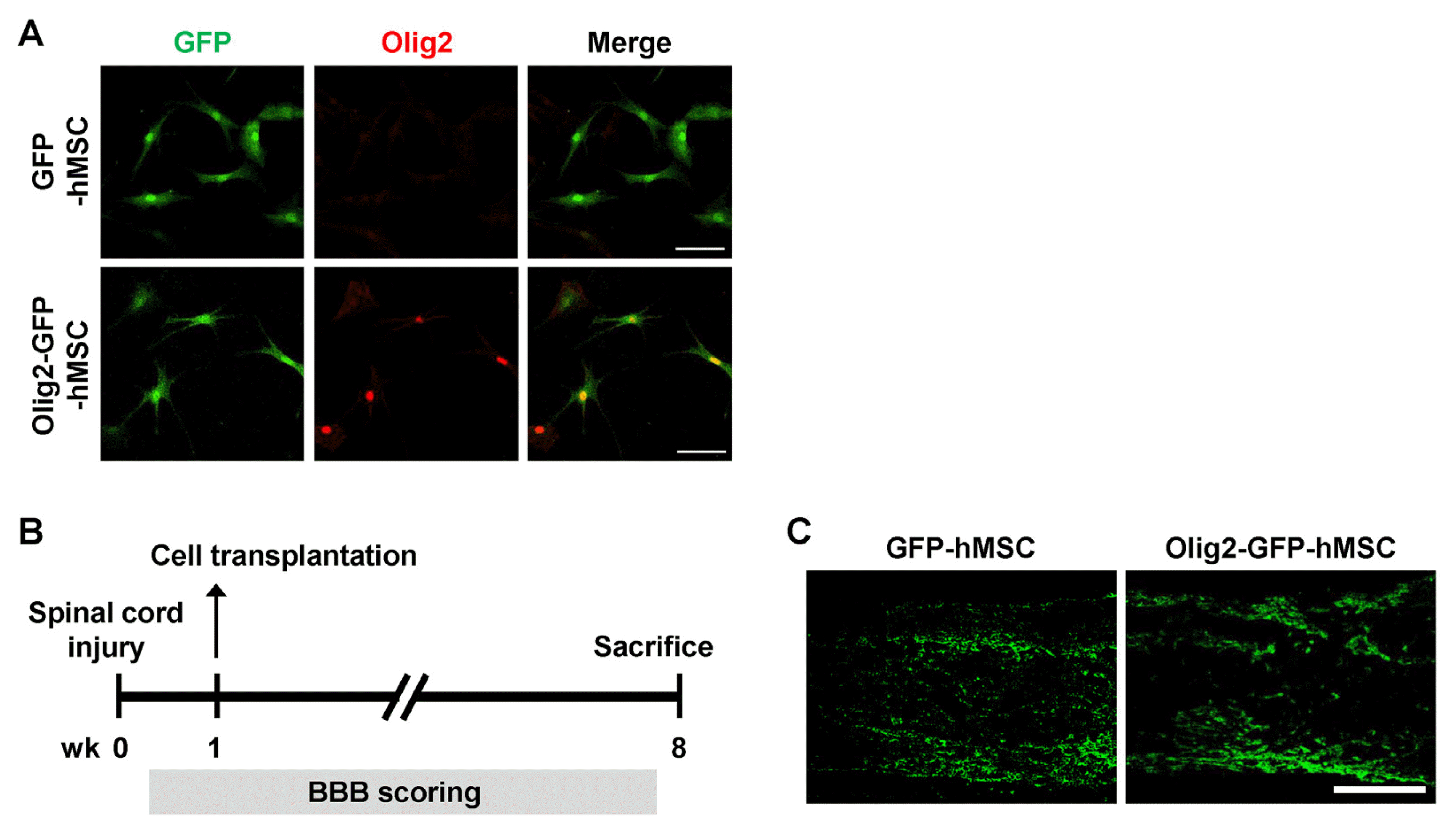 | Fig. 1Survival and distribution of Olig2-GFP-hMSCs in spinal cord lesion after contusive SCI. (A) Green fluorescent protein (GFP) and Olig2 expression in genetically modified hMSCs. The GFP-infected (GFP-hMSCs) and Olig2-GFP-infected (Olig2-GFP-hMSCs) hMSCs were immunostained with an anti-Olig2 (red) antibody. Colocalization of Oilg2 and GFP in the nucleus of Olig2-GFP-hMSCs is shown in the merged image (yellow). Scale bars: 100 μm. (B) Schematic diagram of the experimental design. (C) Immunohistochemistry of transplanted hMSCs in spinal cord lesion at 7 weeks after transplantation. Longitudinal spinal cord sections were immunostained for GFP at 7 weeks after transplantation of GFP-hMSCs and Olig2-GFP-hMSCs. Scale bars: 500 μm. The left side is rostral and the superior side is dorsal. |
Behavioral analysis of locomotor function
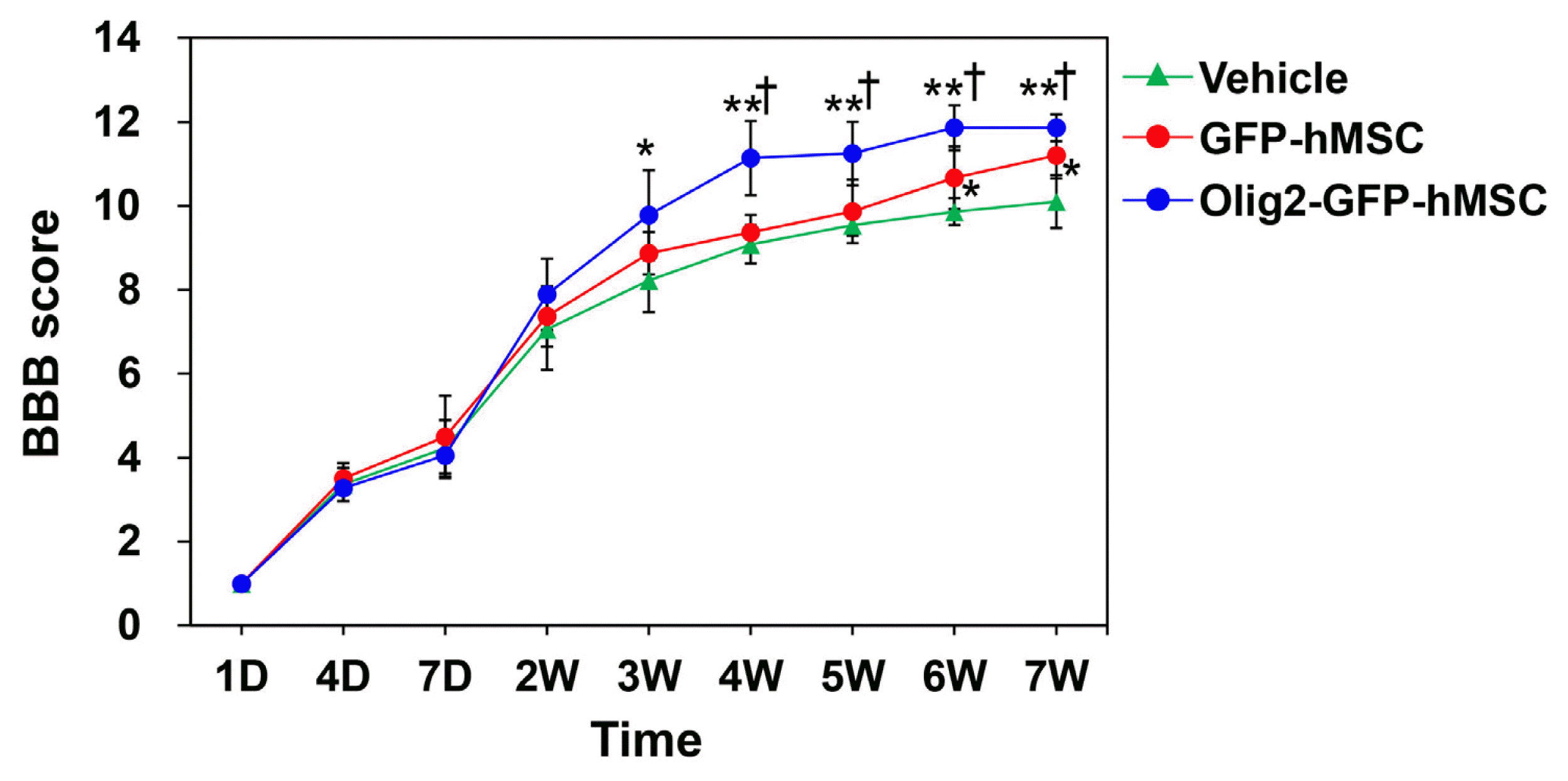 | Fig. 2Behavioral analysis of locomotor function. Effect of transplantation of Olig2-GFP-hMSCs on functional recovery after SCI. BBB locomotor scores of rats with SCI before and after transplantation of vehicle (n=5), GFP-hMSCs (n=5) and Olig2-GFP-hMSCs (n=6) were analyzed. Arrow indicates the time point when transplantation was performed. Data are shown as mean±SEM. *p<0.05 vs. vehicle transplant group, †p<0.05 vs. GFP-hMSC transplant group. |
Histological analysis
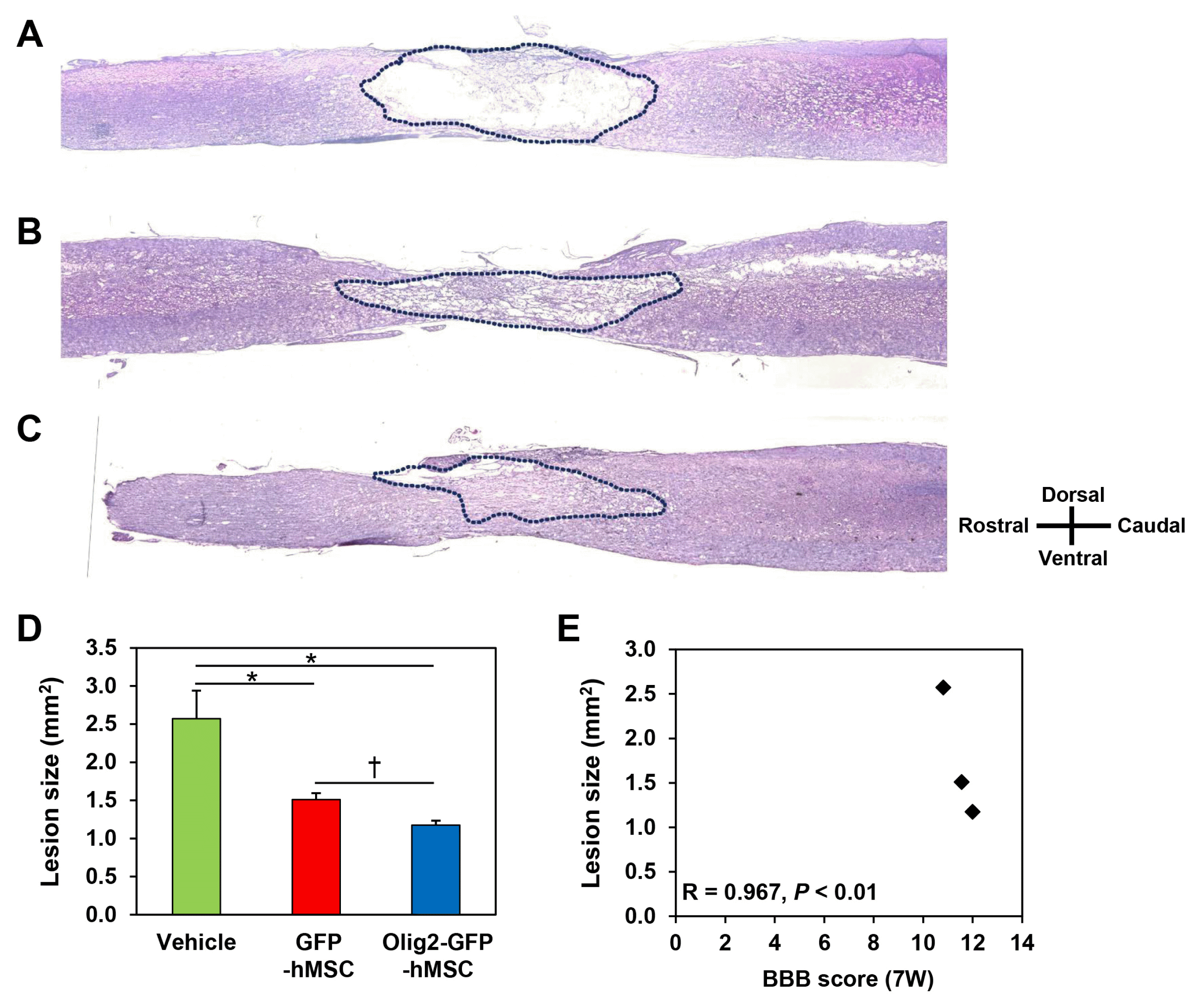 | Fig. 3Morphometry of spinal cord lesion after contusion SCI. Longitudinal spinal cord sections from the vehicle transplant group (A), GFP-hMSC transplant group (B), and Olig2-GFP-hMSC transplant group (C) were stained with hematoxylin and eosin (H&E) at 8 weeks after SCI. Histological analyses were conducted of longitudinal sections encompassing the lesion area. Dotted line outlines the lesion boundary. (D) The histograms represent measurements of the lesion area of vehicle transplant group, GFP-hMSC transplant group and Olig2-GFP-hMSC transplant group. (E) Correlations between BBB score 7 weeks post-injury and lesion size in the spinal cord tissue. Data are shown as mean±SEM. The results are representative of at least three rats in each group. *p<0.05 vs. vehicle transplant group, †p<0.05 vs. GFP-hMSC transplant group. |
Immunohistochemical analysis
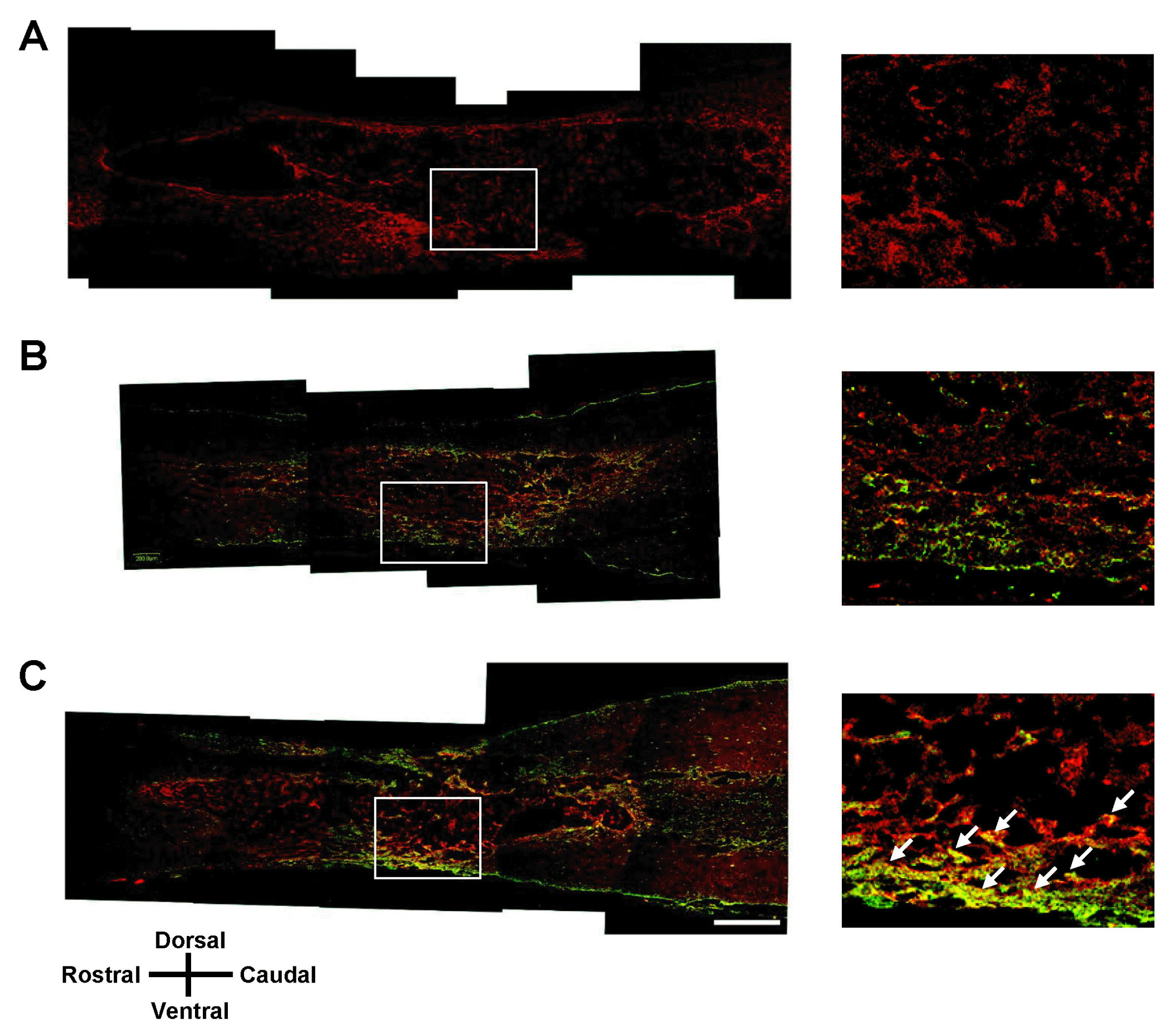 | Fig. 4Olig1 immunohistochemistry of the spinal cord lesion at 7 weeks after transplantation. Longitudinal spinal cord sections were immunostained for Olig1 and GFP 7 weeks after transplantation of vehicle (A), GFP-hMSCs (B) and Olig2-GFP-hMSCs (C). The right panel is magnified images of the boxed area. White arrows show colocalization of Olig1 to GFP-positive cells. Scale bars: 500 μm. In all images, the left side is rostral and the superior side is dorsal. |
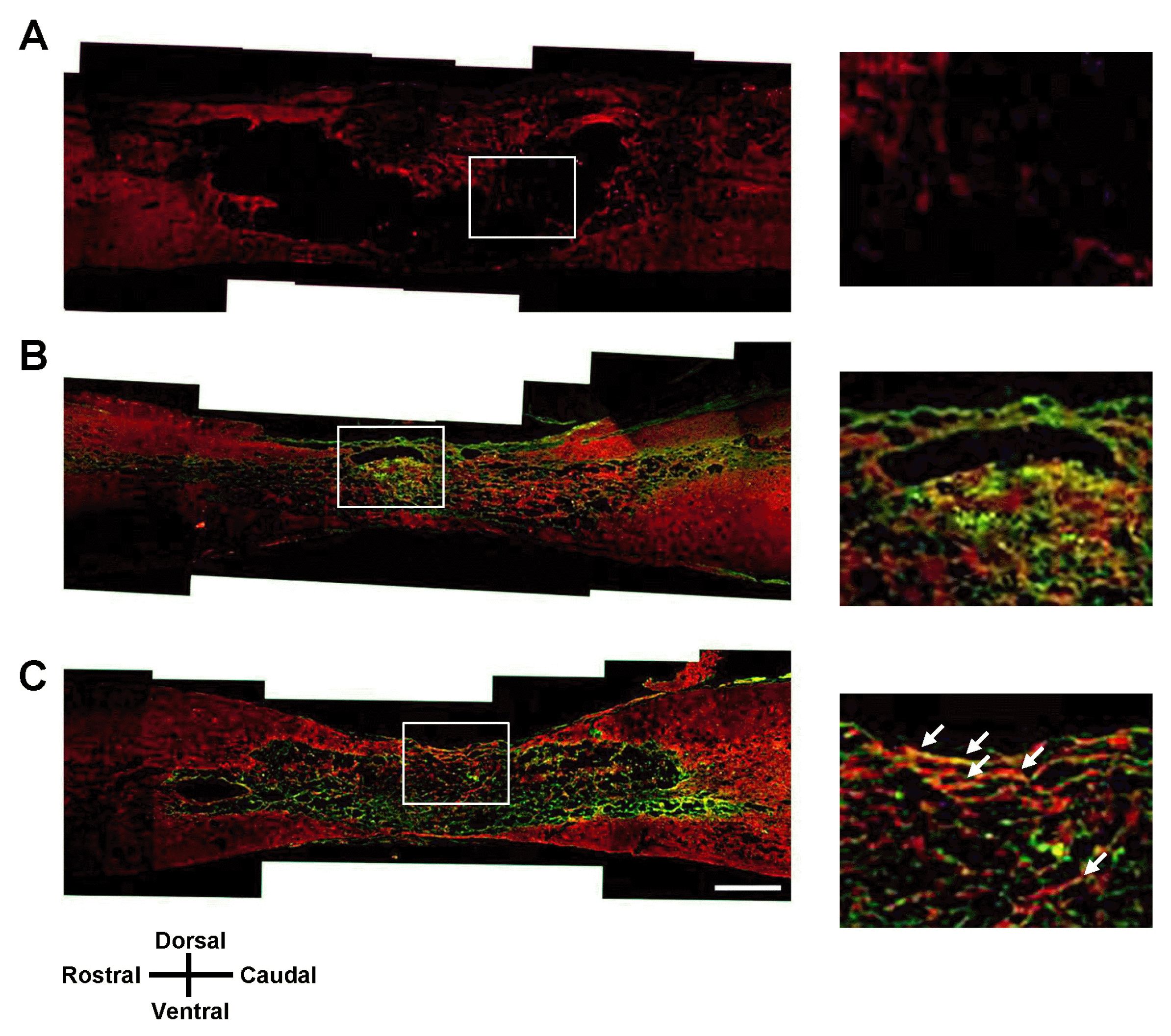 | Fig. 5NF-M immunohistochemistry of the spinal cord lesion at 7 weeks after transplantation. Longitudinal spinal cord sections were immunostained for NF-M and GFP 7 weeks after transplantation of vehicle (A), GFP-hMSCs (B), and Olig2-GFP-hMSCs (C). The right panel shows magnified images of the boxed area. White arrows show colocalization of NF-M to GFP-positive cells. Scale bars: 500 μm. In all images, the left side is rostral and the superior side is dorsal. |
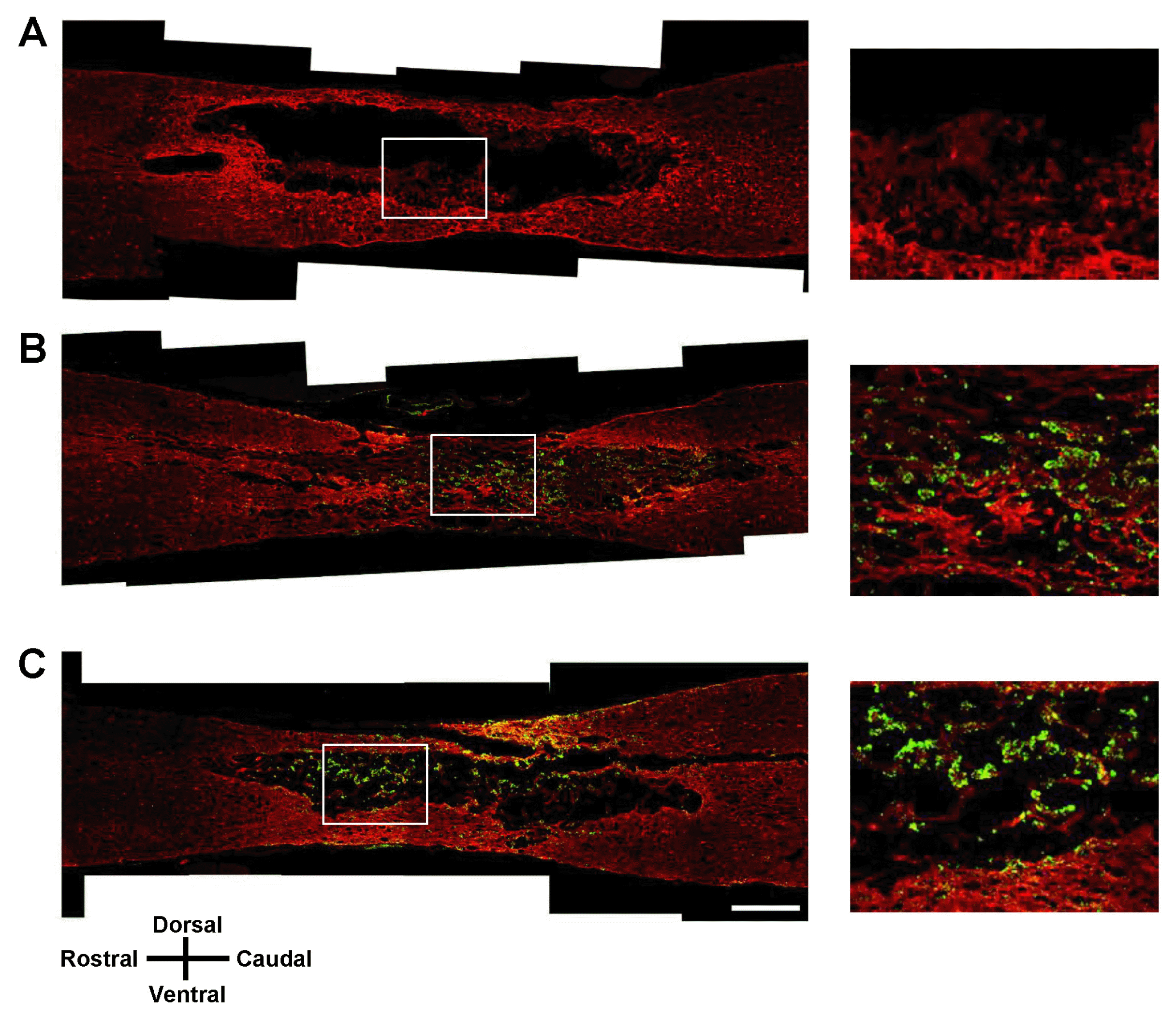 | Fig. 6Iba1 immunohistochemistry of the spinal cord lesion 7 weeks after transplantation. Longitudinal spinal cord sections were immunostained for Iba1 and GFP 7 weeks after transplantation of vehicle (A), GFP-hMSCs (B), and Olig2-GFP-hMSCs (C). The right panel shows magnified images of the boxed area. Scale bars: 500 μm. In all images, the left side is rostral and the superior side is dorsal. |
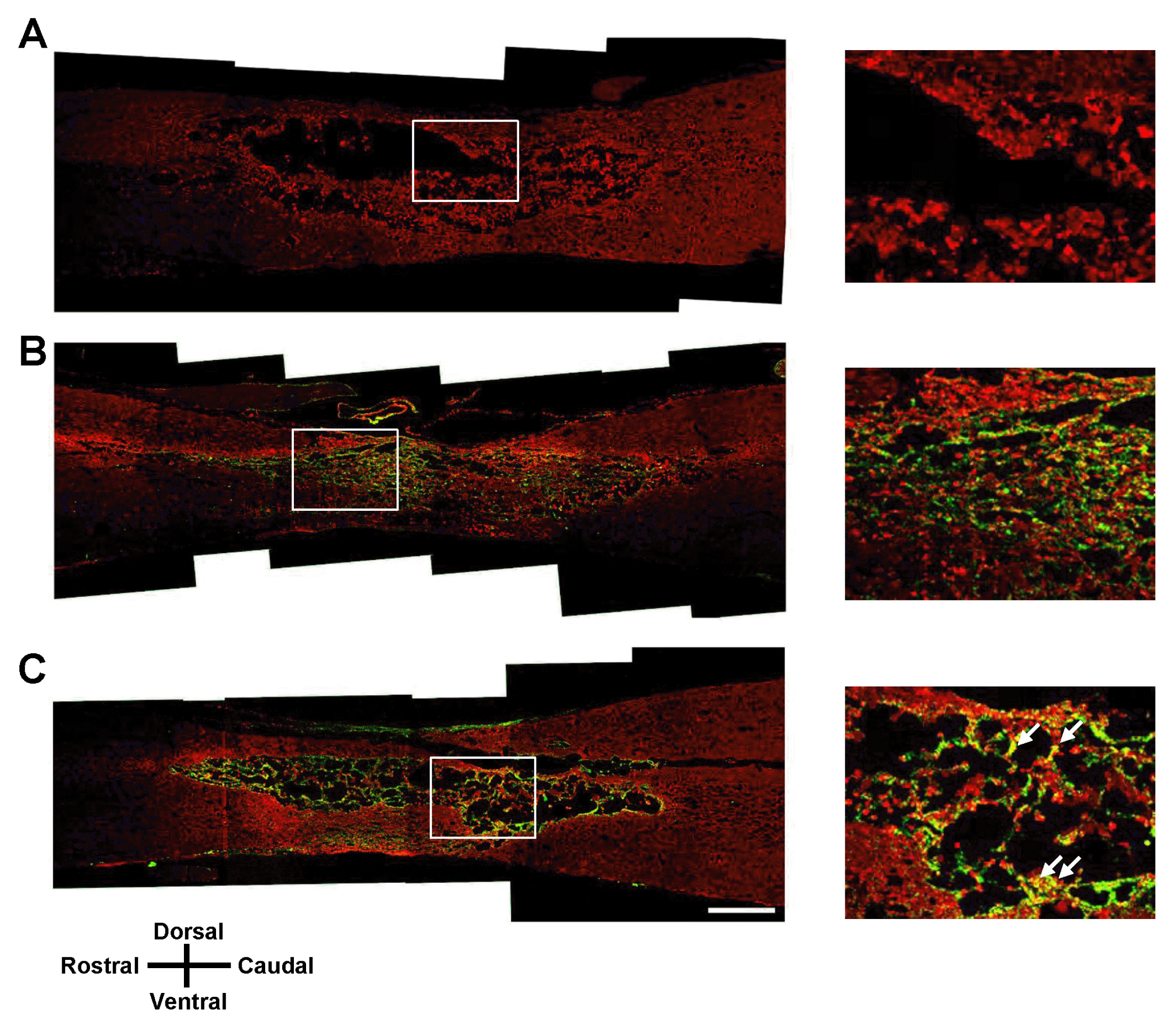 | Fig. 7GFAP immunohistochemistry of the spinal cord lesion at 7 weeks after transplantation. Longitudinal spinal cord sections were immunostained for GFAP and GFP 7 weeks after transplantation of vehicle (A), GFP-hMSCs (B), and Olig2-GFP-hMSCs (C). The right panel shows magnified images of the boxed area. White arrows show colocalization of GFAP to GFP-positive cells. Scale bars: 500 μm. In all images, the left side is rostral and the superior side is dorsal. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download