2. Coll-Bonfill N, de la Cruz-Thea B, Pisano MV, Musri MM. Noncoding RNAs in smooth muscle cell homeostasis: implications in phenotypic switch and vascular disorders. Pflugers Arch. 2016; 468:1071–1087. DOI:
10.1007/s00424-016-1821-x. PMID:
27109570.

3. Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015; 214:33–50. DOI:
10.1111/apha.12466.

5. Prudente A, Favaro WJ, Reis LO, Riccetto CL. Nitric oxide coating polypropylene mesh increases angiogenesis and reduces inflammatory response and apoptosis. Int Urol Nephrol. 2017; 49:597–605. DOI:
10.1007/s11255-017-1520-3. PMID:
28181115.

6. Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009; 54:668–675. DOI:
10.1161/HYPERTENSIONAHA.109.133397. PMID:
19597040. PMCID:
2745434.

8. Nickel KF, Laux V, Heumann R, von Degenfeld G. Thrombin has biphasic effects on the nitric oxide-cGMP pathway in endothelial cells and contributes to experimental pulmonary hypertension. PLoS One. 2013; 8:e63504. DOI:
10.1371/journal.pone.0063504. PMID:
23785394. PMCID:
3681801.

9. Goto J, Ishikawa K, Kawamura K, Watanabe Y, Matumoto H, Sugawara D, Maruyama Y. Heme oxygenase-1 reduces murine monocrotaline-induced pulmonary inflammatory responses and resultant right ventricular overload. Antioxid Redox Signal. 2002; 4:563–568. DOI:
10.1089/15230860260220058. PMID:
12230867.

10. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009; 18:683–692. DOI:
10.1089/scd.2008.0253.

11. Nasri F, Mohtasebi MS, Hashemi E, Zarrabi M, Gholijani N, Sarvestani EK. Therapeutic efficacy of mesenchymal stem cells and mesenchymal stem cells-derived neural progenitors in experimental autoimmune encephalomyelitis. Int J Stem Cells. 2018; 11:68–77. DOI:
10.15283/ijsc17052. PMID:
29699380. PMCID:
5984060.

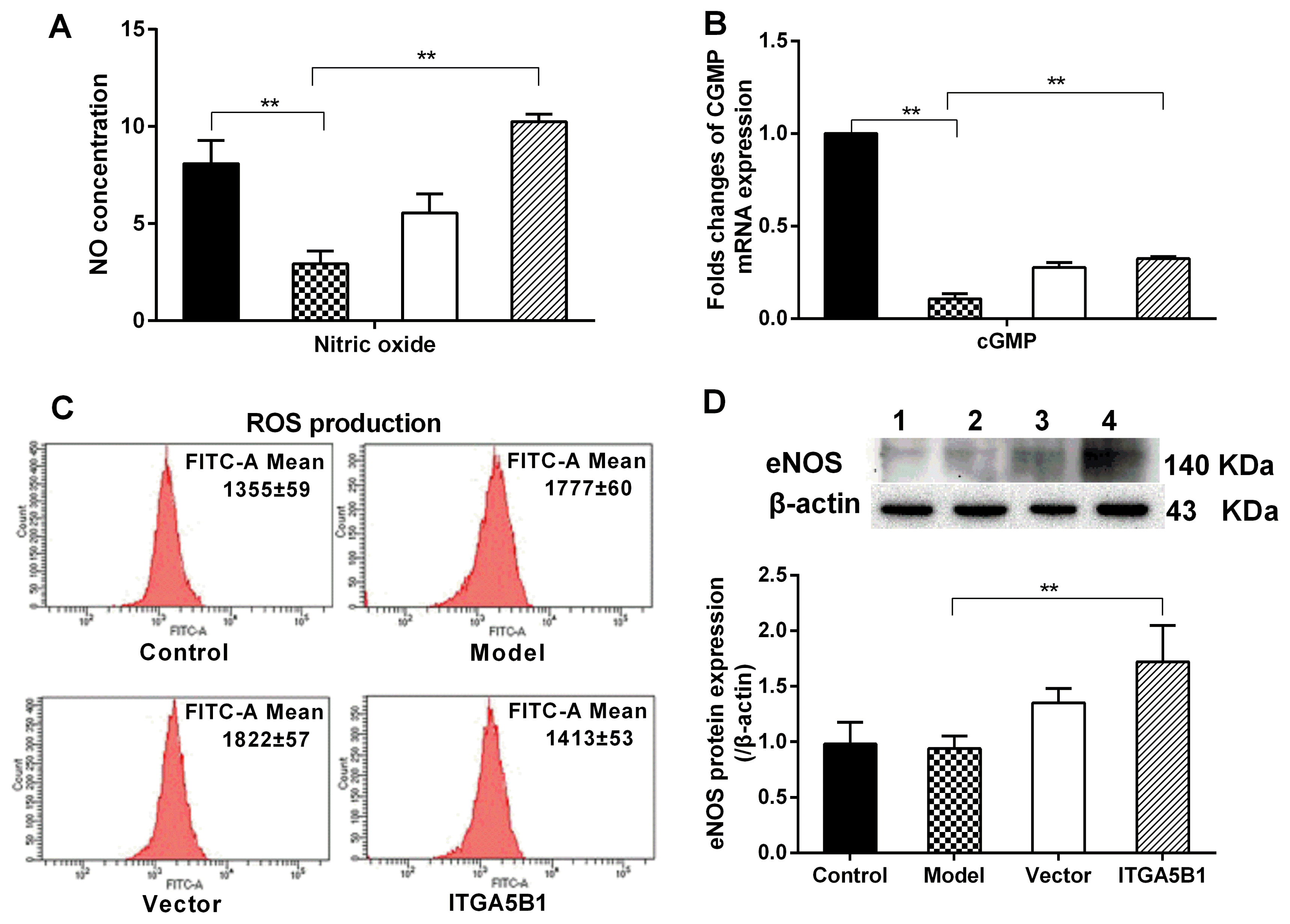
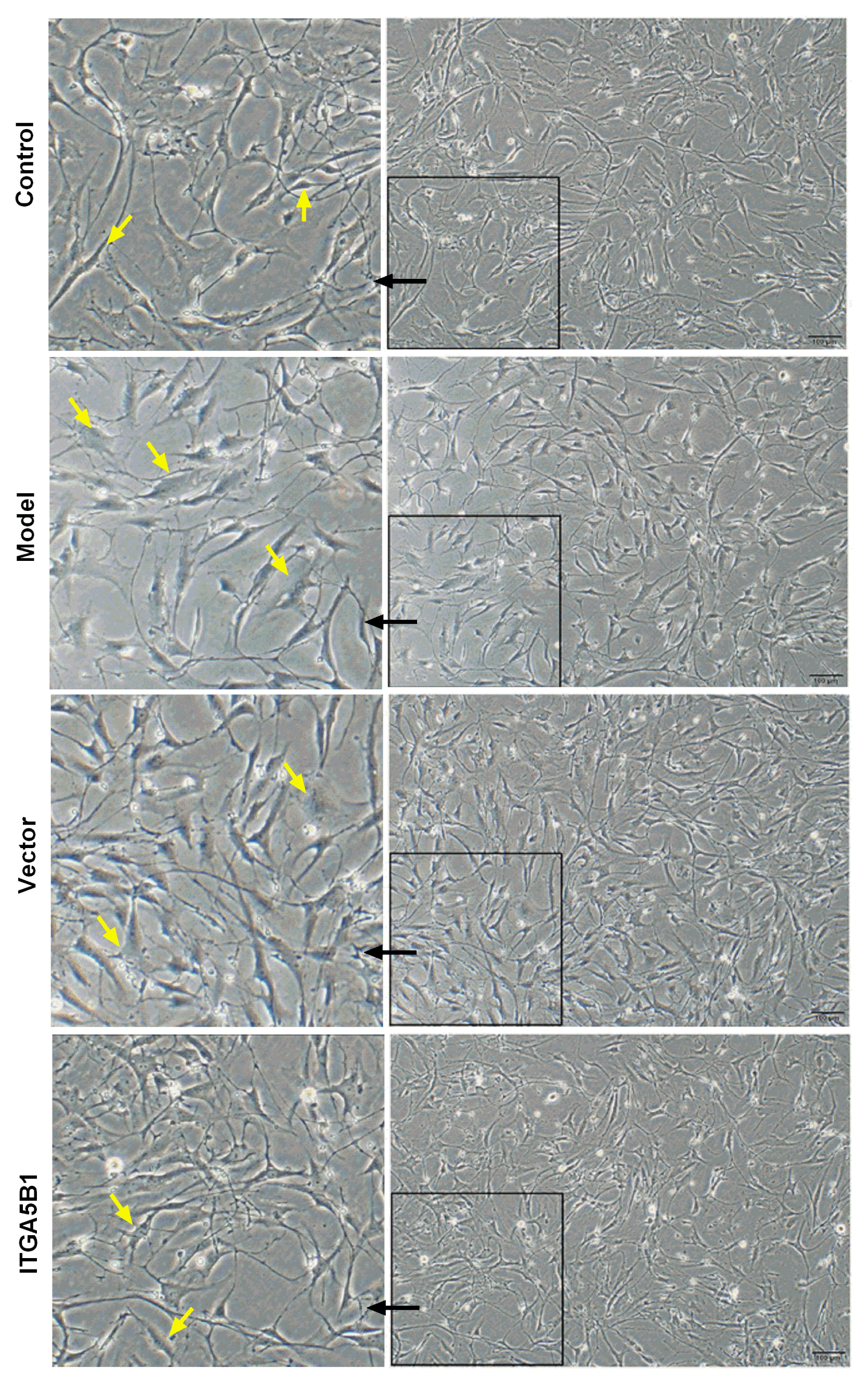
12. Chen HY, Pan L, Yang HL, Xia P, Yu WC, Tang WQ, Zhang YX, Chen SF, Xue YZ, Wang LX. Integrin alpha5beta1 suppresses rBMSCs anoikis and promotes nitric oxide production. Biomed Pharmacother. 2018; 99:1–8. DOI:
10.1016/j.biopha.2018.01.038. PMID:
29324307.

13. Chen H, Yang H, Yue H, Strappe PM, Xia P, Pan L, Zhang Y, Chai S, Chen S, Ma L, Wang L. Mesenchymal Stem Cells Expressing eNOS and a Cav1 Mutant Inhibit Vascular Smooth Muscle Cell Proliferation in a Rat Model of Pulmonary Hypertension. Heart Lung Circ. 2017; 26:509–518. DOI:
10.1016/j.hlc.2016.08.002.

14. Xia P, Chen HY, Chen SF, Wang L, Strappe PM, Yang HL, Zhou CH, Zhang X, Zhang YX, Ma LL, Wang L. The stimulatory effects of eNOS/F92A-Cav1 on NO production and angiogenesis in BMSCs. Biomed Pharmacother. 2016; 77:7–13. DOI:
10.1016/j.biopha.2015.11.001. PMID:
26796258.

15. Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015; 2015:632902. DOI:
10.1155/2015/632902.

16. Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release. 2012; 163:161–169. DOI:
10.1016/j.jconrel.2012.08.031. PMID:
22974832. PMCID:
3495982.

17. Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci. 2006; 11:356–367. DOI:
10.2741/1803.

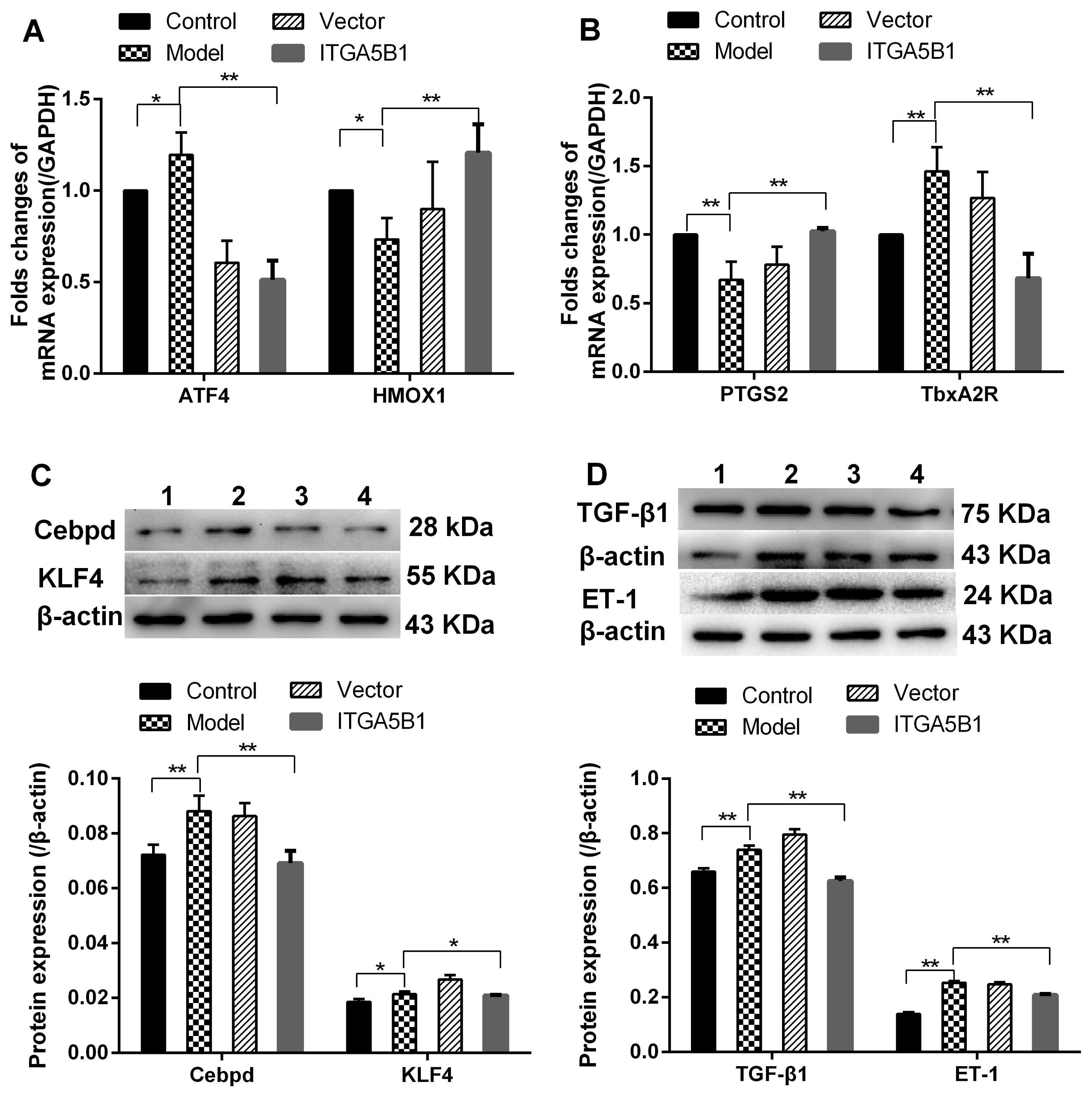
19. Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005; 280:38247–38258. DOI:
10.1074/jbc.M509378200. PMID:
16169848.

20. Autieri MV. Kruppel-like factor 4: transcriptional regulator of proliferation, or inflammation, or differentiation, or all three? Circ Res. 2008; 102:1455–1457. DOI:
10.1161/CIRCRESAHA.108.178954. PMID:
18566311. PMCID:
2754858.
21. Huang S, Chen P, Shui X, He Y, Wang H, Zheng J, Zhang L, Li J, Xue Y, Chen C, Lei W. Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J Pharm Pharmacol. 2014; 66:1469–1477. DOI:
10.1111/jphp.12273. PMID:
24835111.

22. Iwasaki Y, Suganami T, Hachiya R, Shirakawa I, Kim-Saijo M, Tanaka M, Hamaguchi M, Takai-Igarashi T, Nakai M, Miyamoto Y, Ogawa Y. Activating transcription factor 4 links metabolic stress to interleukin-6 expression in macrophages. Diabetes. 2014; 63:152–161. DOI:
10.2337/db13-0757.

23. Belhaj A, Dewachter L, Kerbaul F, Brimioulle S, Dewachter C, Naeije R, Rondelet B. Heme oxygenase-1 and inflammation in experimental right ventricular failure on prolonged overcirculation-induced pulmonary hypertension. PLoS One. 2013; 8:e69470. DOI:
10.1371/journal.pone.0069470. PMID:
23936023. PMCID:
3723896.

24. Tamosiuniene R, Manouvakhova O, Mesange P, Saito T, Qian J, Sanyal M, Lin YC, Nguyen LP, Luria A, Tu AB, Sante JM, Rabinovitch M, Fitzgerald DJ, Graham BB, Habtezion A, Voelkel NF, Aurelian L, Nicolls MR. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circ Res. 2018; 122:1689–1702. DOI:
10.1161/CIRCRESAHA.117.312058. PMID:
29545367. PMCID:
5993601.

25. Hill MR, Papafili A, Booth H, Lawson P, Hubner M, Beynon H, Read C, Lindahl G, Marshall RP, McAnulty RJ, Laurent GJ. Functional prostaglandin-endoperoxide synthase 2 polymorphism predicts poor outcome in sarcoidosis. Am J Respir Crit Care Med. 2006; 174:915–922. DOI:
10.1164/rccm.200512-1839OC. PMID:
16840740.

26. Peroni JF, Borjesson DL. Anti-inflammatory and immunomodulatory activities of stem cells. Vet Clin North Am Equine Pract. 2011; 27:351–362. DOI:
10.1016/j.cveq.2011.06.003. PMID:
21872763.

27. Lee H, Lee JC, Kwon JH, Kim KC, Cho MS, Yang YS, Oh W, Choi SJ, Seo ES, Lee SJ, Wang TJ, Hong YM. The effect of umbilical cord blood derived mesenchymal stem cells in monocrotaline-induced pulmonary artery hypertension rats. J Korean Med Sci. 2015; 30:576–585. DOI:
10.3346/jkms.2015.30.5.576. PMID:
25931788. PMCID:
4414641.

28. Vaillancourt M, Ruffenach G, Meloche J, Bonnet S. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol. 2015; 31:407–415. DOI:
10.1016/j.cjca.2014.10.023. PMID:
25630876.

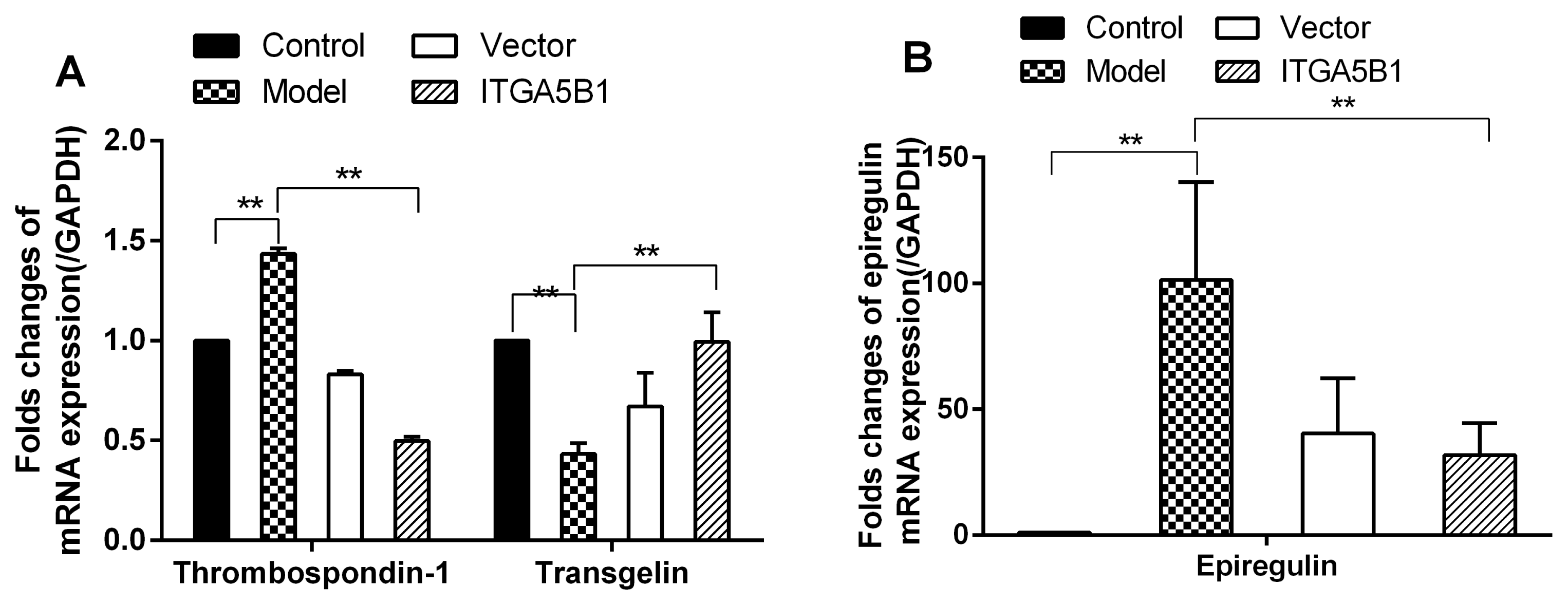
29. Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017; 8:15494. DOI:
10.1038/ncomms15494.

30. Takahashi M, Hayashi K, Yoshida K, Ohkawa Y, Komurasaki T, Kitabatake A, Ogawa A, Nishida W, Yano M, Monden M, Sobue K. Epiregulin as a major autocrine/paracrine factor released from ERK- and p38MAPK-activated vascular smooth muscle cells. Circulation. 2003; 108:2524–2529. DOI:
10.1161/01.CIR.0000096482.02567.8C. PMID:
14581411.

31. Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22α promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res. 2012; 111:685–696. DOI:
10.1161/CIRCRESAHA.112.269811. PMID:
22811558. PMCID:
3517884.

32. Chen H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018; 134:32–37. DOI:
10.1016/j.prostaglandins.2017.11.004.

33. Diehl KJ, Stauffer BL, Dow CA, Bammert TD, Brunjes DL, Greiner JJ, DeSouza CA. Chronic nebivolol treatment suppresses endothelin-1-mediated vasoconstrictor tone in adults with elevated blood pressure. Hypertension. 2016; 67:1196–1204. DOI:
10.1161/HYPERTENSIONAHA.115.06979. PMID:
27113048. PMCID:
4871319.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download