1. da Silva LB, Palma PV, Cury PM, Bueno V. Evaluation of stem cell administration in a model of kidney ischemia-reperfusion injury. Int Immunopharmacol. 2007; 7:1609–1616. DOI:
10.1016/j.intimp.2007.08.014. PMID:
17996670.

2. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005; 11:367–368. DOI:
10.1038/nm0405-367. PMID:
15812508.

3. Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004; 94:678–685. DOI:
10.1161/01.RES.0000118601.37875.AC. PMID:
14739163.

4. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004; 109:1543–1549. DOI:
10.1161/01.CIR.0000124062.31102.57. PMID:
15023891.

5. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation- independent mechanisms. Am J Physiol Renal Physiol. 2005; 289:F31–42. DOI:
10.1152/ajprenal.00007.2005.
6. Wang Y, He J, Pei X, Zhao W. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton). 2013; 18:201–208. DOI:
10.1111/nep.12018.

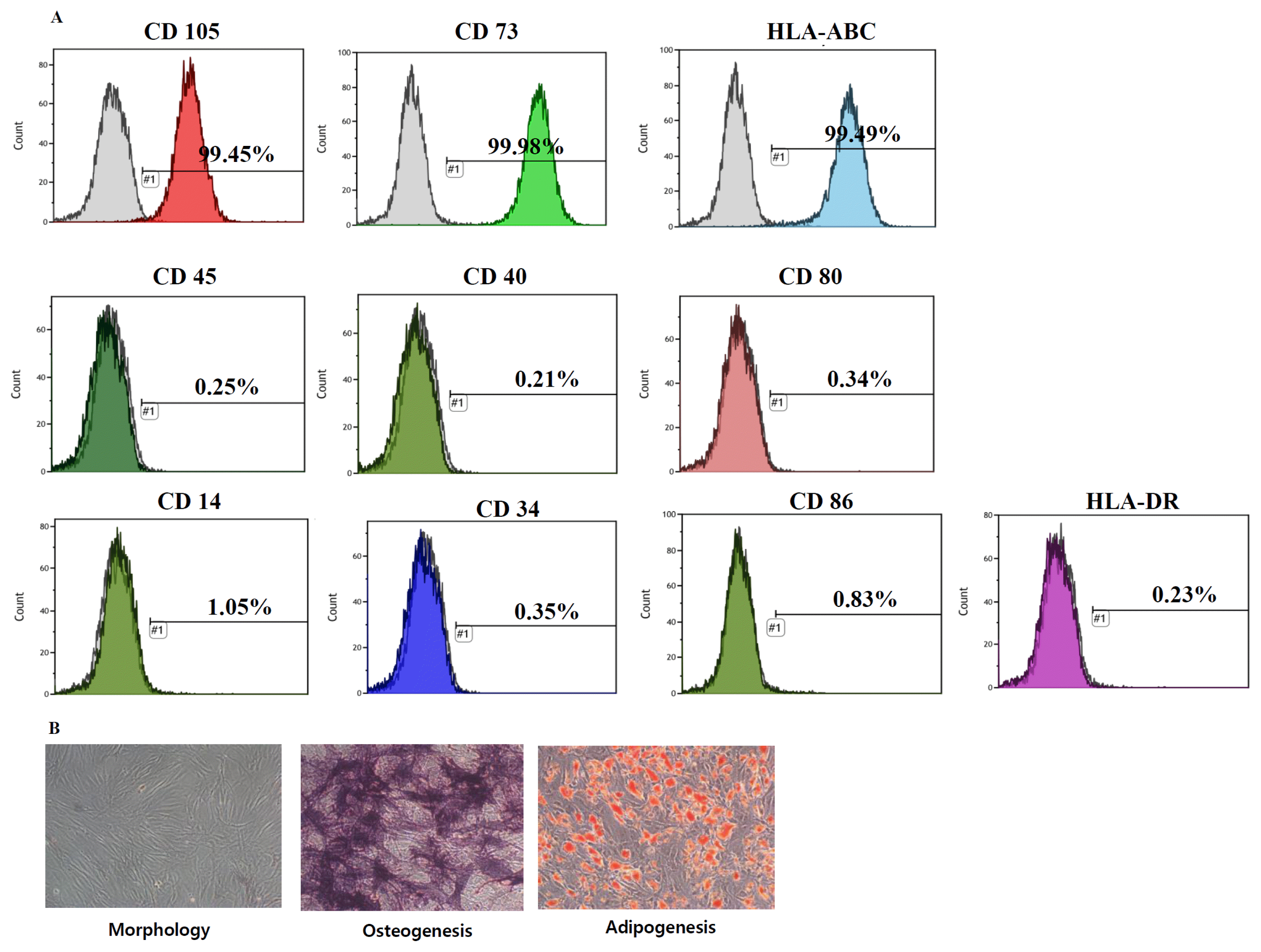
7. Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010; 121:2211–2220. DOI:
10.1161/CIRCULATIONAHA.109.928796. PMID:
20458011. PMCID:
2919223.

8. Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007; 292:F1626–F1635. DOI:
10.1152/ajprenal.00339.2006. PMID:
17213465.

9. Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007; 18:2486–2496. DOI:
10.1681/ASN.2007020140. PMID:
17656474.

10. Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, Reisner Y. Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells. 2006; 24:1185–1193. DOI:
10.1634/stemcells.2005-0265. PMID:
16410390.

12. Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004; 114:5–14. DOI:
10.1172/JCI200422353. PMID:
15232604. PMCID:
437979.

13. Kwon T, Jeong IG, Lee C, You D, Hong B, Hong JH, Ahn H, Kim CS. Acute kidney injury after radical cystectomy for bladder cancer is associated with chronic kidney disease and mortality. Ann Surg Oncol. 2016; 23:686–693. DOI:
10.1245/s10434-015-4886-4.

14. Yang Y, Song M, Liu Y, Liu H, Sun L, Peng Y, Liu F, Venkatachalam MA, Dong Z. Renoprotective approaches and strategies in acute kidney injury. Pharmacol Ther. 2016; 163:58–73. DOI:
10.1016/j.pharmthera.2016.03.015. PMID:
27108948. PMCID:
5123830.

16. Erpicum P, Detry O, Weekers L, Bonvoisin C, Lechanteur C, Briquet A, Beguin Y, Krzesinski JM, Jouret F. Mesenchymal stromal cell therapy in conditions of renal ischaemia/reperfusion. Nephrol Dial Transplant. 2014; 29:1487–1493. DOI:
10.1093/ndt/gft538. PMID:
24516234.

17. Lee C, Jang MJ, Kim BH, Park JY, You D, Jeong IG, Hong JH, Kim CS. Recovery of renal function after administration of adipose-tissue-derived stromal vascular fraction in rat model of acute kidney injury induced by ischemia/reperfusion injury. Cell Tissue Res. 2017; 368:603–613. DOI:
10.1007/s00441-017-2585-0. PMID:
28283911.

18. Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011; 9:51. DOI:
10.1186/1479-5876-9-51. PMID:
21545725. PMCID:
3112438.

19. Yip HK, Chang LT, Wu CJ, Sheu JJ, Youssef AA, Pei SN, Lee FY, Sun CK. Autologous bone marrow-derived mononuclear cell therapy prevents the damage of viable myocardium and improves rat heart function following acute anterior myocardial infarction. Circ J. 2008; 72:1336–1345. DOI:
10.1253/circj.72.1336. PMID:
18654023.

20. Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007; 18:2921–2928. DOI:
10.1681/ASN.2006121318. PMID:
17942965.
21. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005; 68:1613–1617. DOI:
10.1111/j.1523-1755.2005.00573.x. PMID:
16164638.

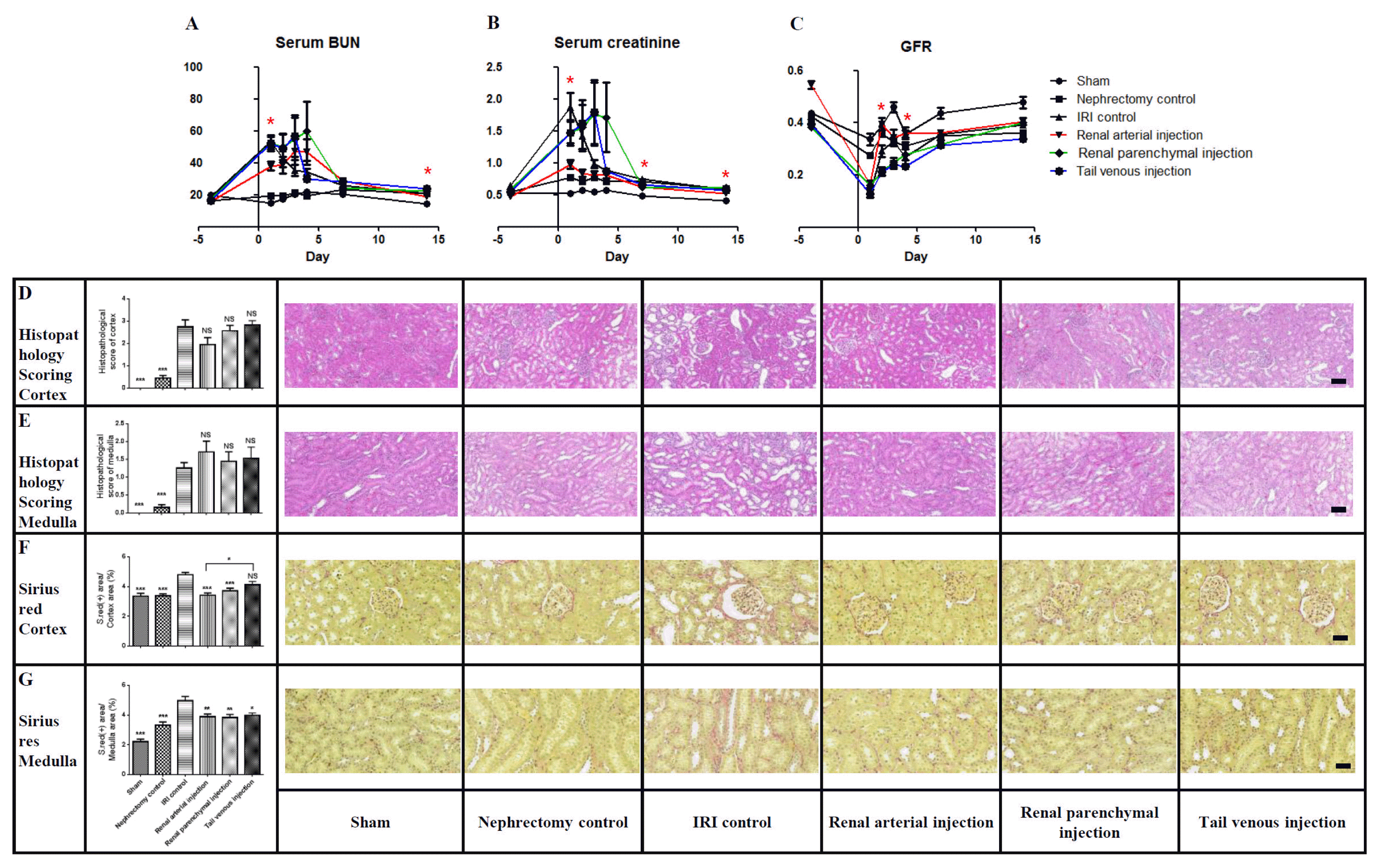
22. Behr L, Hekmati M, Fromont G, Borenstein N, Noel LH, Lelievre-Pegorier M, Laborde K. Intra renal arterial injection of autologous mesenchymal stem cells in an ovine model in the postischemic kidney. Nephron Physiol. 2007; 107:p65–76. DOI:
10.1159/000109821. PMID:
17940346.

23. Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012; 46:635–645. DOI:
10.1016/j.nbd.2012.03.002. PMID:
22426403. PMCID:
3353023.

24. Singh A, Singh A, Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010–2015). Stem Cell Res Ther. 2016; 7:82. DOI:
10.1186/s13287-016-0341-0.
25. Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014; 508:269–273. DOI:
10.1038/nature13034. PMID:
24590072. PMCID:
3984353.

26. Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010; 223:27–35.

27. Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2015; 4:412. DOI:
10.5966/sctm.2013-0196erratum. PMID:
25795657. PMCID:
4367501.

28. Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015; 33:1818–1828. DOI:
10.1002/stem.1976. PMID:
25702874.

29. Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, Lee S, Lim J, Jeong SY, Kwon J, Kim M, Choi SJ, Oh W, Yang YS, Hwang HH, Yu HY, Ryu CM, Jeon HB, Shin DM. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018; May. 22. [Epub]. DOI:
10.1038/s41375-018-0151-8.

30. You D, Jang MJ, Kim BH, Choi KR, Lee C, Song G, Shin HC, Jeong IG, Suh N, Kim YM, Ahn TY, Kim CS. Bone marrow-derived mesenchymal stromal cell therapy in a rat model of cavernous nerve injury: preclinical study for approval. Cytotherapy. 2016; 18:870–880. DOI:
10.1016/j.jcyt.2016.04.010. PMID:
27260208.

31. Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002; 110:1083–1091. DOI:
10.1172/JCI0215623. PMID:
12393844. PMCID:
150794.

32. Cai J, Yu X, Xu R, Fang Y, Qian X, Liu S, Teng J, Ding X. Maximum efficacy of mesenchymal stem cells in rat model of renal ischemia-reperfusion injury: renal artery administration with optimal numbers. PLoS One. 2014; 9:e92347. DOI:
10.1371/journal.pone.0092347. PMID:
24637784. PMCID:
3956922.

33. Peired AJ, Sisti A, Romagnani P. Mesenchymal stem cell-based therapy for kidney disease: a review of clinical evidence. Stem Cells Int. 2016; 2016:4798639. DOI:
10.1155/2016/4798639. PMID:
27721835. PMCID:
5046016.

34. Zhu XY, Lerman A, Lerman LO. Concise review: mesenchymal stem cell treatment for ischemic kidney disease. Stem Cells. 2013; 31:1731–1736. DOI:
10.1002/stem.1449. PMID:
23766020. PMCID:
3795813.

35. Bianchi F, Sala E, Donadei C, Capelli I, La Manna G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J Stem Cells. 2014; 6:644–650. DOI:
10.4252/wjsc.v6.i5.644. PMID:
25426262. PMCID:
4178265.

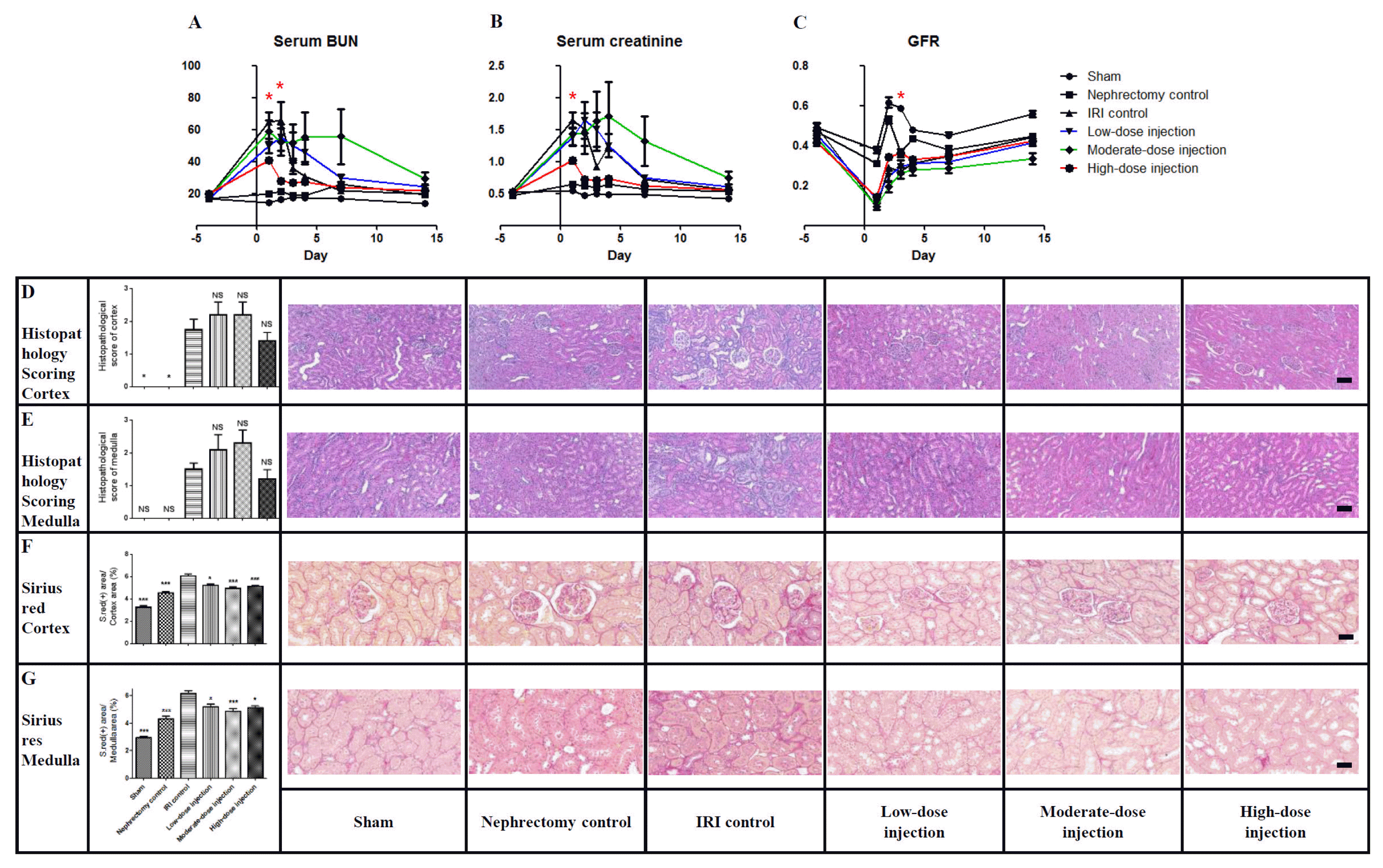
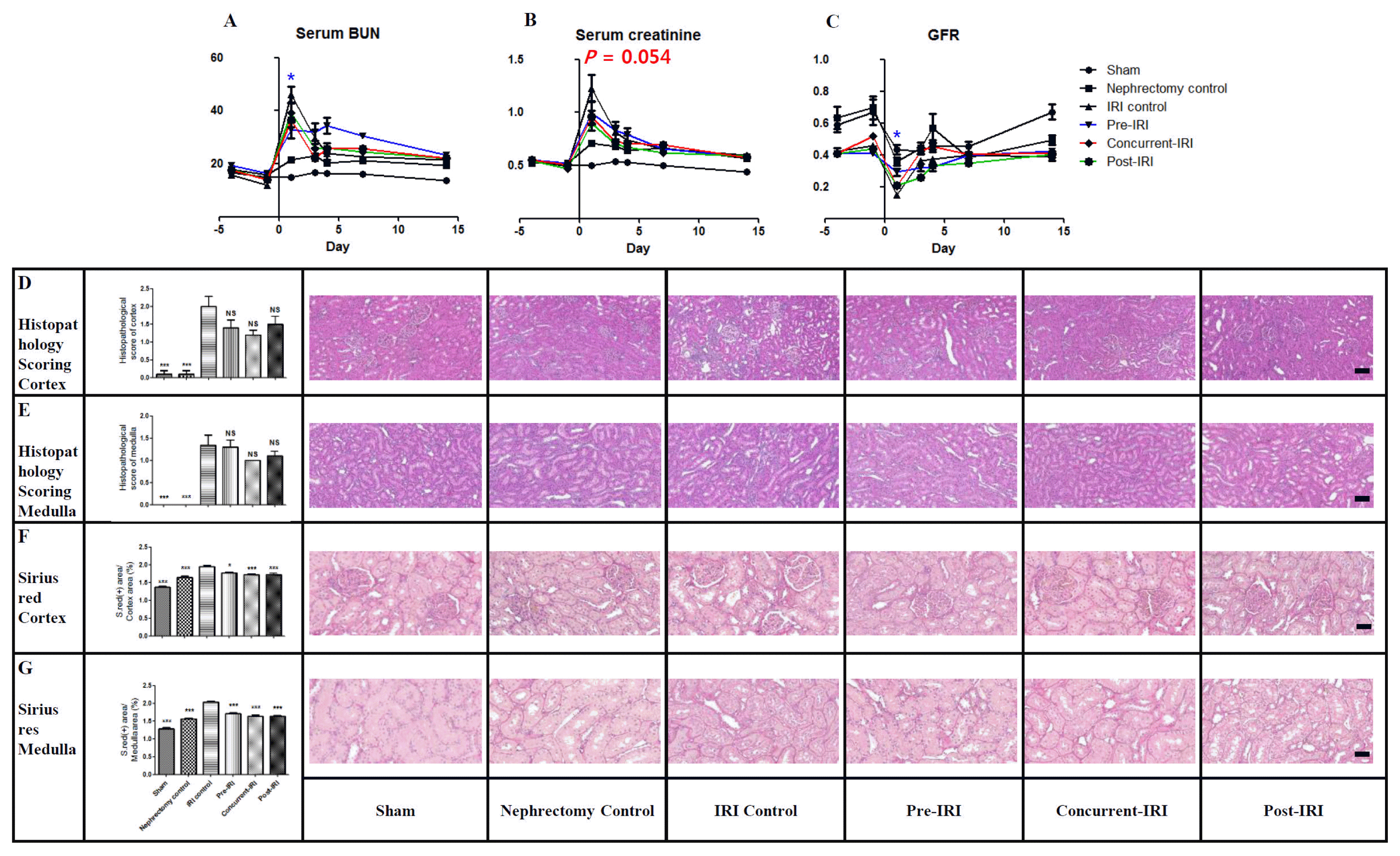
36. Liu X, Cai J, Jiao X, Yu X, Ding X. Therapeutic potential of mesenchymal stem cells in acute kidney injury is affected by administration timing. Acta Biochim Biophys Sin (Shanghai). 2017; 49:338–348. DOI:
10.1093/abbs/gmx016.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download