|
Proteins only in 12 hr differentiation |
|
sp|P04406|G3P_HUMAN |
Glyceraldehyde-3-phosphate dehydrogenase |
0.8479277 |
0.0043422 |
0.8963611 |
0.0539365 |
1.29605 |
0.0126654 |
|
sp|P06733|ENOA_HUMAN |
Alpha-enolase |
0.8004785 |
0.003791 |
0.8899997 |
0.0494512 |
0.8673062 |
0.0889979 |
|
sp|Q16658|FSCN1_HUMAN |
Fascin |
0.8632113 |
0.0081369 |
0.9985458 |
0.9725319 |
0.9803382 |
0.7522615 |
|
sp|P62258|1433E_HUMAN |
14-3-3 protein epsilon |
0.7345669 |
0.0014579 |
0.7889716 |
0.0540487 |
2.7000389 |
1.51E-08 |
|
sp|P63244|GBLP_HUMAN |
Guanine nucleotide-binding protein subunit beta-2-like 1 |
0.7850113 |
0.0023291 |
1.026148 |
0.7182648 |
0.9216409 |
0.2950645 |
|
sp|P08238|HS90B_HUMAN |
Heat shock protein HSP 90-beta |
0.7536395 |
0.0140655 |
0.860231 |
0.1114735 |
0.9950555 |
0.9490238 |
|
sp|P62701|RS4X_HUMAN |
40S ribosomal protein S4, X isoform |
0.7593142 |
0.0005935 |
1.144268 |
0.2589218 |
1.1194659 |
0.2733781 |
|
sp|P09651|ROA1_HUMAN |
Heterogeneous nuclear ribonucleoprotein A1 |
0.7305018 |
0.0051622 |
1.106936 |
0.3859383 |
2.0843611 |
0.0006238 |
|
sp|P61247|RS3A_HUMAN |
40S ribosomal protein S3a |
0.836074 |
0.049875 |
1.03742 |
0.4289024 |
1.320195 |
0.001181 |
|
sp|Q9Y617|SERC_HUMAN |
Phosphoserine aminotransferase |
0.7837498 |
0.0029119 |
0.8880233 |
0.135478 |
1.087113 |
0.2114475 |
|
sp|P30041|PRDX6_HUMAN |
Peroxiredoxin-6 |
0.7284337 |
0.0064425 |
0.7679175 |
0.0504089 |
1.132565 |
0.0761582 |
|
sp|P63104|1433Z_HUMAN |
14-3-3 protein zeta/delta |
0.7794408 |
0.0424038 |
0.8261315 |
0.1741135 |
1.9782979 |
3.104E-05 |
|
sp|P08865|RSSA_HUMAN |
40S ribosomal protein SA |
0.7462529 |
0.0099222 |
1.036908 |
0.5412839 |
0.9988793 |
0.9859492 |
|
sp|P62937|PPIA_HUMAN |
Peptidyl-prolyl cis-trans isomerase A |
0.7663312 |
0.0020865 |
1.062439 |
0.3747487 |
2.0133979 |
0.0001145 |
|
sp|Q13347|EIF3I_HUMAN |
Eukaryotic translation initiation factor 3 subunit I |
0.8121918 |
0.0102658 |
0.9462903 |
0.3618241 |
1.018685 |
0.8342772 |
|
sp|O75534|CSDE1_HUMAN |
Cold shock domain-containing protein E1 |
0.771526 |
0.0034917 |
0.9900259 |
0.8615462 |
0.9455857 |
0.6485646 |
|
sp|P30050|RL12_HUMAN |
60S ribosomal protein L12 |
0.849311 |
0.0232884 |
1.0797631 |
0.2055826 |
0.8181012 |
0.0908025 |
|
sp|P55786|PSA_HUMAN |
Puromycin-sensitive aminopeptidase |
0.4095355 |
0.0208944 |
0.8498592 |
0.2518263 |
1.940941 |
0.0009965 |
|
sp|P02511|CRYAB_HUMAN |
Alpha-crystallin B chain |
0.6343505 |
0.0032148 |
1.02856 |
0.685686 |
1.220765 |
0.0158245 |
|
sp|P30044|PRDX5_HUMAN |
Peroxiredoxin-5, mitochondrial |
0.7028021 |
0.0389368 |
0.8285266 |
0.1052497 |
0.8535319 |
0.1997427 |
|
sp|P62328|TYB4_HUMAN |
Thymosin beta-4 |
0.4920007 |
0.0332198 |
0.8621768 |
0.2507035 |
1.239386 |
0.4872846 |
|
sp|P63220|RS21_HUMAN |
40S ribosomal protein S21 |
0.7481042 |
0.0084351 |
1.052633 |
0.6723817 |
0.8575014 |
0.073444 |
|
sp|P18085|ARF4_HUMAN |
ADP-ribosylation factor 4 |
0.6821533 |
0.0280265 |
0.9178579 |
0.79254 |
0.8463399 |
0.4097828 |
|
sp|P18621|RL17_HUMAN |
60S ribosomal protein L17 |
0.6151012 |
0.0349046 |
1.057725 |
0.5479587 |
0.6039299 |
0.0704918 |
|
sp|P16949|STMN1_HUMAN |
Stathmin |
0.8414626 |
0.0448395 |
0.985819 |
0.8424742 |
2.1492679 |
1.178E-05 |
|
sp|Q9NR30|DDX21_HUMAN |
Nucleolar RNA helicase 2 |
0.743278 |
0.0099099 |
0.9631584 |
0.8492183 |
0.5311144 |
0.0828007 |
|
sp|P49773|HINT1_HUMAN |
Histidine triad nucleotide-binding protein 1 |
0.7960001 |
0.0157561 |
0.9326981 |
0.5084993 |
1.8662699 |
0.0207274 |
|
sp|Q96JY6|PDLI2_HUMAN |
PDZ and LIM domain protein 2 |
0.7705169 |
0.0181184 |
0.8789578 |
0.3594843 |
0.5489806 |
0.0511937 |
|
sp|P09669|COX6C_HUMAN |
Cytochrome c oxidase subunit 6C |
0.6379359 |
0.013377 |
1.116226 |
0.2616933 |
0.9925451 |
0.9516448 |
|
sp|P30520|PURA2_HUMAN |
Adenylosuccinate synthetase isozyme 2 |
0.7602439 |
0.0369208 |
0.9035071 |
0.2785547 |
0.8973022 |
0.4219353 |
|
sp|P06493|CDK1_HUMAN |
Cyclin-dependent kinase 1 |
0.7085927 |
0.0163401 |
0.7424781 |
0.1200113 |
2.3315251 |
0.0133933 |
|
sp|P00403|COX2_HUMAN |
Cytochrome c oxidase subunit 2 |
0.5474085 |
0.0229093 |
0.8213091 |
0.1686617 |
1.686554 |
0.0307677 |
|
sp|Q9H910|HN1L_HUMAN |
Hematological and neurological expressed 1-like protein |
0.7686939 |
0.0395484 |
0.9141212 |
0.2472908 |
1.1195869 |
0.4445185 |
|
sp|Q13308|PTK7_HUMAN |
Inactive tyrosine-protein kinase 7 |
0.7176057 |
0.0155243 |
0.7806843 |
0.2195718 |
0.8202866 |
0.2265889 |
|
sp|Q8N3F8|MILK1_HUMAN |
MICAL-like protein 1 |
0.6290908 |
0.0317877 |
0.9276105 |
0.4822235 |
1.50721 |
0.0750358 |
|
sp|Q96K17|BT3L4_HUMAN |
Transcription factor BTF3 homolog 4 |
0.6892197 |
0.0486006 |
0.6607736 |
0.1085802 |
0.496424 |
0.112247 |
|
Common Proteins in 12 and 24 hr differentiation |
|
sp|P06744|G6PI_HUMAN |
Glucose-6-phosphate isomerase |
0.7193677 |
0.0006254 |
0.6672136 |
8.246E-05 |
2.2487891 |
1.036E-08 |
|
sp|Q9NZU5|LMCD1_HUMAN |
LIM and cysteine-rich domains protein 1 |
0.7071645 |
0.0004705 |
0.6401113 |
4.341E-08 |
2.296283 |
2.267E-09 |
|
sp|P60174|TPIS_HUMAN |
Triosephosphate isomerase |
0.7501674 |
0.0113529 |
0.7045917 |
0.0004007 |
2.5271809 |
6.364E-08 |
|
sp|P49588|SYAC_HUMAN |
Alanine--tRNA ligase, cytoplasmic |
0.6795498 |
5.394E-05 |
0.5746737 |
3.913E-06 |
1.371278 |
4.908E-05 |
|
sp|P04792|HSPB1_HUMAN |
Heat shock protein beta-1 |
0.5604951 |
0.0001762 |
0.7426677 |
0.0209271 |
1.541172 |
0.0089136 |
|
sp|P35222|CTNB1_HUMAN |
Catenin beta-1 |
0.8595872 |
0.0128006 |
0.7611314 |
1.582E-05 |
0.8387557 |
0.1275463 |
|
sp|P36871|PGM1_HUMAN |
Phosphoglucomutase-1 |
0.7463383 |
0.0001896 |
0.6675955 |
6.797E-05 |
0.9217962 |
0.1677606 |
|
sp|Q15417|CNN3_HUMAN |
Calponin-3 |
0.5205765 |
0.0011651 |
0.5401105 |
0.0003287 |
3.3978031 |
1.723E-06 |
|
sp|P62979|RS27A_HUMAN |
Ubiquitin-40S ribosomal protein S27a |
0.5051308 |
0.0128211 |
0.6933423 |
0.0414675 |
1.412729 |
0.1300915 |
|
sp|P35613|BASI_HUMAN |
Basigin |
0.7667562 |
0.0006819 |
0.786752 |
0.0052719 |
1.478635 |
8.056E-05 |
|
sp|P00966|ASSY_HUMAN |
Argininosuccinate synthase |
0.6765513 |
0.0031044 |
0.6359119 |
0.0020193 |
2.331908 |
3.533E-06 |
|
sp|Q12841|FSTL1_HUMAN |
Follistatin-related protein 1 |
0.7639553 |
0.0345735 |
0.5640365 |
0.0190533 |
1.070824 |
0.5687613 |
|
sp|P07437|TBB5_HUMAN |
Tubulin beta chain |
0.4618195 |
0.0499191 |
0.5135489 |
0.0062117 |
0.9821569 |
0.9072029 |
|
sp|P17936|IBP3_HUMAN |
Insulin-like growth factor-binding protein 3 |
0.5418074 |
0.0067458 |
0.4080258 |
0.0063709 |
0.3198267 |
0.0523204 |
|
sp|P50579|AMPM2_HUMAN |
Methionine aminopeptidase 2 |
0.7693873 |
0.0483193 |
0.6800565 |
0.0174458 |
0.938477 |
0.4942554 |
|
sp|O15427|MOT4_HUMAN |
Monocarboxylate transporter 4 |
0.6468069 |
0.0497313 |
0.6395898 |
0.0477522 |
0.1273622 |
0.0785593 |
|
sp|Q9BV57|MTND_HUMAN |
1,2-dihydroxy-3-keto-5-methylthiopentene dioxygenase |
0.5140472 |
0.002501 |
0.4079044 |
0.004841 |
2.0234301 |
0.0111199 |
|
sp|Q92896|GSLG1_HUMAN |
Golgi apparatus protein 1 |
0.6584018 |
0.0078619 |
0.5889162 |
0.0004774 |
0.8151252 |
0.1259657 |
|
sp|P28300|LYOX_HUMAN |
Protein-lysine 6-oxidase |
0.6829536 |
0.0262415 |
0.3348025 |
0.0257906 |
0.3427074 |
0.226561 |
|
Proteins only in 24 hr differentiation |
|
sp|Q16881|TRXR1_HUMAN |
Thioredoxin reductase 1, cytoplasmic |
1.0364439 |
0.3616807 |
0.8219741 |
0.0005493 |
0.9360311 |
0.1332849 |
|
sp|P48735|IDHP_HUMAN |
Isocitrate dehydrogenase (NADP), mitochondrial |
1.187354 |
0.001626 |
0.760787 |
0.0022276 |
4.9582028 |
3.073E-11 |
|
sp|P17858|K6PL_HUMAN |
6-phosphofructokinase, liver type |
0.8796898 |
0.4738032 |
0.8036854 |
0.0026158 |
0.7909461 |
0.1816241 |
|
sp|P49411|EFTU_HUMAN |
Elongation factor Tu, mitochondrial |
1.136111 |
0.0326502 |
0.7871462 |
0.0011592 |
1.947157 |
6.357E-07 |
|
sp|P20810|ICAL_HUMAN |
Calpastatin |
0.8750533 |
0.1081493 |
0.7568965 |
0.0196435 |
0.7850653 |
0.1937321 |
|
sp|P30086|PEBP1_HUMAN |
Phosphatidylethanolamine-binding protein 1 |
0.8731124 |
0.0211942 |
0.8283793 |
0.0038564 |
3.1945789 |
1.385E-07 |
|
sp|P35237|SPB6_HUMAN |
Serpin B6 |
0.8802679 |
0.1745626 |
0.8257262 |
0.0086406 |
0.9815652 |
0.7505975 |
|
sp|P07339|CATD_HUMAN |
Cathepsin D |
1.051528 |
0.4394175 |
0.8192881 |
0.0117687 |
1.546684 |
0.0010593 |
|
sp|Q92900|RENT1_HUMAN |
Regulator of nonsense transcripts 1 |
0.9178929 |
0.2185951 |
0.8558482 |
0.027399 |
0.8075969 |
0.0586372 |
|
sp|P09960|LKHA4_HUMAN |
Leukotriene A-4 hydrolase |
0.9437257 |
0.5129562 |
0.8397065 |
0.049417 |
1.160228 |
0.2741844 |
|
sp|Q16822|PCKGM_HUMAN |
Phosphoenolpyruvate carboxykinase (GTP), mitochondrial |
0.9528816 |
0.8248823 |
0.5842852 |
0.0001355 |
0.6504104 |
0.0637888 |
|
sp|Q8N8S7|ENAH_HUMAN |
Protein enabled homolog |
0.8274177 |
0.0571576 |
0.6830815 |
0.0069704 |
0.9318953 |
0.5751261 |
|
sp|P07203|GPX1_HUMAN |
Glutathione peroxidase 1 |
0.8624336 |
0.2153537 |
0.7866376 |
0.0186332 |
2.587467 |
0.001527 |
|
sp|Q99961|SH3G1_HUMAN |
Endophilin-A2 |
0.8050464 |
0.0544368 |
0.8494079 |
0.0429622 |
0.8313006 |
0.2537356 |
|
sp|P63092|GNAS2_HUMAN |
Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
0.8557481 |
0.1282786 |
0.8180471 |
0.0324584 |
1.7606061 |
0.0025444 |
|
sp|P68400|CSK21_HUMAN |
Casein kinase II subunit alpha |
0.9240049 |
0.2656185 |
0.8457134 |
0.0444661 |
1.105554 |
0.3466572 |
|
sp|Q9BRK5|CAB45_HUMAN |
45 kDa calcium-binding protein |
0.5650094 |
0.1218079 |
0.5348625 |
0.0083942 |
1.283482 |
0.052236 |
|
sp|P08648|ITA5_HUMAN |
Integrin alpha-5 |
0.8827975 |
0.283567 |
0.8334129 |
0.0390296 |
0.2592828 |
0.1002662 |
|
sp|Q04760|LGUL_HUMAN |
Lactoylglutathione lyase |
0.8773558 |
0.1461125 |
0.767711 |
0.031137 |
2.81745 |
0.0119689 |
|
sp|P25325|THTM_HUMAN |
3-mercaptopyruvate sulfurtransferase |
0.9139439 |
0.4899827 |
0.6435751 |
0.0176827 |
1.033338 |
0.784981 |
|
sp|Q8IVL6|P3H3_HUMAN |
Prolyl 3-hydroxylase 3 |
0.8073167 |
0.2868669 |
0.5731455 |
0.0264081 |
0.3668924 |
0.0672391 |
|
sp|Q9HB07|MYG1_HUMAN |
UPF0160 protein MYG1, mitochondrial |
0.8819175 |
0.6004307 |
0.622142 |
0.0089968 |
1.078177 |
0.7573638 |
|
sp|P10619|PPGB_HUMAN |
Lysosomal protective protein |
0.8569001 |
0.4883563 |
0.6964424 |
0.0469068 |
0.9313151 |
0.868605 |
|
sp|P52888|THOP1_HUMAN |
Thimet oligopeptidase |
0.9622395 |
0.7386975 |
0.837072 |
0.0478316 |
1.31484 |
0.3072187 |
|
sp|O96013|PAK4_HUMAN |
Serine/threonine-protein kinase PAK 4 |
1.0217381 |
0.8920609 |
0.6765835 |
0.0410042 |
1.333773 |
0.5003013 |
|
sp|Q8ND76|CCNY_HUMAN |
Cyclin-Y |
0.8020011 |
0.216727 |
0.65859 |
0.0363463 |
0.8060961 |
0.4418439 |
|
Common Proteins in 24 hr differentiation and GBCs |
|
sp|P02751|FINC_HUMAN |
Fibronectin |
1.05277 |
0.1802123 |
0.4407711 |
5.836E-19 |
0.1742529 |
1.563E-18 |
|
sp|Q01995|TAGL_HUMAN |
Transgelin |
0.6692728 |
0.0876543 |
0.6856616 |
0.0109215 |
0.0669862 |
0.0001268 |
|
sp|O43795|MYO1B_HUMAN |
Unconventional myosin-Ib |
1.024282 |
0.5635512 |
0.8692154 |
0.0026827 |
0.3533631 |
3.264E-06 |
|
sp|Q9NZN4|EHD2_HUMAN |
EH domain-containing protein 2 |
0.9012447 |
0.0739072 |
0.65933 |
0.0001831 |
0.2320304 |
5.896E-06 |
|
sp|Q07954|LRP1_HUMAN |
Prolow-density lipoprotein receptor-related protein 1 |
0.8714587 |
0.0038484 |
0.6779363 |
5.012E-08 |
0.7306147 |
0.0002078 |
|
sp|Q9H4M9|EHD1_HUMAN |
EH domain-containing protein 1 |
0.9417256 |
0.2532193 |
0.8380113 |
0.000365 |
0.5321978 |
5.697E-06 |
|
sp|P80723|BASP1_HUMAN |
Brain acid soluble protein 1 |
0.9116059 |
0.1819695 |
0.7202799 |
0.0410783 |
0.0676923 |
1.521E-07 |
|
sp|P04844|RPN2_HUMAN |
Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 2 |
0.9017079 |
0.2878655 |
0.8192101 |
0.0399242 |
0.4075336 |
0.0022847 |
|
sp|P30837|AL1B1_HUMAN |
Aldehyde dehydrogenase X, mitochondrial |
0.9417027 |
0.3682509 |
0.670727 |
0.001719 |
0.3164505 |
0.0001068 |
|
sp|Q6WCQ1|MPRIP_HUMAN |
Myosin phosphatase Rho-interacting protein |
0.9359979 |
0.2837241 |
0.801344 |
0.0029636 |
0.5354406 |
0.0075895 |
|
sp|Q15063|POSTN_HUMAN |
Periostin |
1.500139 |
0.0001878 |
0.5885563 |
0.0010843 |
0.3422702 |
0.0043296 |
|
sp|P54727|RD23B_HUMAN |
UV excision repair protein RAD23 homolog B |
0.8896854 |
0.1443552 |
0.7950544 |
0.0151775 |
0.6107698 |
0.0002073 |
|
sp|P68032|ACTC_HUMAN |
Actin, alpha cardiac muscle 1 |
0.5544822 |
0.1149586 |
0.5408995 |
0.0260236 |
0.3912214 |
0.0114004 |
|
sp|P98082|DAB2_HUMAN |
Disabled homolog 2 |
0.9615206 |
0.6100879 |
0.7754198 |
0.0185135 |
0.3850327 |
0.0200069 |
|
sp|P12111|CO6A3_HUMAN |
Collagen alpha-3 (VI) chain |
1.334669 |
0.0003428 |
0.8098702 |
0.0016986 |
0.3830687 |
0.0017061 |
|
sp|Q969G5|PRDBP_HUMAN |
Protein kinase C delta-binding protein |
0.9411212 |
0.5362816 |
0.8662521 |
0.049586 |
0.2927489 |
6.347E-05 |
|
sp|P62070|RRAS2_HUMAN |
Ras-related protein R-Ras2 |
0.8131321 |
0.1026654 |
0.7656406 |
0.04193 |
0.4747174 |
0.0066609 |
|
sp|Q96KP4|CNDP2_HUMAN |
Cytosolic non-specific dipeptidase |
0.9173274 |
0.410962 |
0.7831527 |
0.0147257 |
0.7192879 |
0.0329394 |
|
sp|Q15758|AAAT_HUMAN |
Neutral amino acid transporter B(0) |
1.041995 |
0.5513352 |
0.8404037 |
0.0433146 |
0.6372434 |
0.0378501 |
|
sp|Q15582|BGH3_HUMAN |
Transforming growth factor-beta-induced protein ig-h3 |
0.9638007 |
0.7168077 |
0.4342145 |
0.0015891 |
0.193604 |
0.0083622 |
|
sp|Q9Y570|PPME1_HUMAN |
Protein phosphatase methylesterase 1 |
0.8078368 |
0.0714756 |
0.7711414 |
0.0079941 |
0.6471239 |
0.0051838 |
|
sp|Q9NRV9|HEBP1_HUMAN |
Heme-binding protein 1 |
0.7728173 |
0.053967 |
0.7515048 |
0.024625 |
0.2896793 |
0.0215531 |
|
sp|Q9UBG0|MRC2_HUMAN |
C-type mannose receptor 2 |
0.7810244 |
0.1127253 |
0.6724297 |
0.0199727 |
0.3234429 |
0.01084 |
|
sp|O14495|LPP3_HUMAN |
Lipid phosphate phosphohydrolase 3 |
0.7414259 |
0.1132276 |
0.711651 |
0.0106689 |
0.1977974 |
0.0078443 |
|
sp|P08473|NEP_HUMAN |
Neprilysin |
0.8780729 |
0.3033772 |
0.6565581 |
0.0029237 |
0.2834384 |
0.0031473 |
|
sp|O00461|GOLI4_HUMAN |
Golgi integral membrane protein 4 |
0.9204073 |
0.406372 |
0.7727606 |
0.0378564 |
0.7051854 |
0.0251325 |
|
sp|Q16270|IBP7_HUMAN |
Insulin-like growth factor-binding protein 7 |
0.340861 |
0.0689417 |
0.3130495 |
0.0357396 |
0.1773682 |
0.0483662 |
|
Common Proteins in 12 hr differentiation and GBCs |
|
sp|P35579|MYH9_HUMAN |
Myosin-9 |
0.8423561 |
1.212E-07 |
0.9585411 |
0.204614 |
0.1792233 |
1.745E-39 |
|
sp|P14618|KPYM_HUMAN |
Pyruvate kinase isozymes M1/M2 |
0.7591904 |
0.001561 |
0.9149806 |
0.1026978 |
0.6729065 |
5.251E-05 |
|
sp|P13639|EF2_HUMAN |
Elongation factor 2 |
0.7993308 |
2.653E-05 |
0.9536575 |
0.2111206 |
0.6309205 |
9.996E-09 |
|
sp|P30101|PDIA3_HUMAN |
Protein disulfide-isomerase A3 |
0.7731736 |
8.563E-05 |
1.065763 |
0.2355234 |
0.5595611 |
4.566E-10 |
|
sp|Q07065|CKAP4_HUMAN |
Cytoskeleton-associated protein 4 |
0.8585722 |
0.0056568 |
1.09707 |
0.0344548 |
0.6766971 |
1.092E-06 |
|
sp|P09493|TPM1_HUMAN |
Tropomyosin alpha-1 chain |
0.3823238 |
0.0089529 |
0.7206582 |
0.1853321 |
0.1964161 |
0.0019547 |
|
sp|P36578|RL4_HUMAN |
60S ribosomal protein L4 |
0.7818362 |
0.0121028 |
0.8841913 |
0.1588947 |
0.5938498 |
0.00011 |
|
sp|Q15084|PDIA6_HUMAN |
Protein disulfide-isomerase A6 |
0.8253382 |
0.0353444 |
1.105119 |
0.1535215 |
0.5150038 |
0.000791 |
|
sp|P67936|TPM4_HUMAN |
Tropomyosin alpha-4 chain |
0.6354412 |
0.0050757 |
0.9631136 |
0.873189 |
0.4140534 |
0.0004444 |
|
sp|Q9NR12|PDLI7_HUMAN |
PDZ and LIM domain protein 7 |
0.8502656 |
0.0033457 |
0.9716707 |
0.7257622 |
0.2132873 |
4.529E-06 |
|
sp|P62424|RL7A_HUMAN |
60S ribosomal protein L7a |
0.6631073 |
0.0090768 |
0.9861412 |
0.8999533 |
0.6202911 |
0.0028769 |
|
sp|Q9ULV4|COR1C_HUMAN |
Coronin-1C |
0.8000384 |
0.003047 |
0.8927166 |
0.0218495 |
0.2160703 |
2.519E-07 |
|
sp|P26641|EF1G_HUMAN |
Elongation factor 1-gamma |
0.7462852 |
0.0186326 |
1.047043 |
0.4670203 |
0.6336192 |
0.0009937 |
|
sp|P18124|RL7_HUMAN |
60S ribosomal protein L7 |
0.7980775 |
0.0165588 |
1.04485 |
0.6165355 |
0.5023291 |
8.299E-05 |
|
sp|Q02878|RL6_HUMAN |
60S ribosomal protein L6 |
0.7674008 |
0.0030989 |
0.8946929 |
0.0621624 |
0.5335739 |
3.795E-06 |
|
sp|O15144|ARPC2_HUMAN |
Actin-related protein 2/3 complex subunit 2 |
0.7838175 |
0.0044124 |
0.9419952 |
0.5304531 |
0.5372279 |
6.401E-05 |
|
sp|P60660|MYL6_HUMAN |
Myosin light polypeptide 6 |
0.7616986 |
0.0495097 |
1.068398 |
0.2886685 |
0.5832632 |
0.0147406 |
|
sp|Q15293|RCN1_HUMAN |
Reticulocalbin-1 |
0.6665307 |
0.0002087 |
0.8986852 |
0.0508759 |
0.1602958 |
4.174E-08 |
|
sp|Q96HC4|PDLI5_HUMAN |
PDZ and LIM domain protein 5 |
0.5892135 |
0.0008179 |
0.8770573 |
0.5314806 |
0.2118111 |
5.798E-06 |
|
sp|P46781|RS9_HUMAN |
40S ribosomal protein S9 |
0.7164086 |
0.0056955 |
1.013286 |
0.8541168 |
0.6084469 |
0.0008322 |
|
sp|P62241|RS8_HUMAN |
40S ribosomal protein S8 |
0.723499 |
0.008818 |
1.1233 |
0.1691011 |
0.7230386 |
0.0046592 |
|
sp|Q8TDX7|NEK7_HUMAN |
Serine/threonine-protein kinase Nek7 |
0.8028638 |
0.0005725 |
0.8820205 |
0.0219638 |
0.2457737 |
5.175E-07 |
|
sp|P63241|IF5A1_HUMAN |
Eukaryotic translation initiation factor 5A-1 |
0.804385 |
0.0316132 |
1.133515 |
0.2812248 |
0.5861024 |
0.0029435 |
|
sp|P24844|MYL9_HUMAN |
Myosin regulatory light polypeptide 9 |
0.771409 |
0.0317043 |
0.845117 |
0.0967934 |
0.2045051 |
0.0099072 |
|
sp|P39019|RS19_HUMAN |
40S ribosomal protein S19 |
0.6352447 |
0.0016217 |
1.0988491 |
0.2598861 |
0.6985727 |
0.0125732 |
|
sp|P62249|RS16_HUMAN |
40S ribosomal protein S16 |
0.858236 |
0.0042911 |
1.211066 |
0.0024016 |
0.7129009 |
1.012E-05 |
|
sp|P21291|CSRP1_HUMAN |
Cysteine and glycine-rich protein 1 |
0.5783014 |
0.0076937 |
0.7048385 |
0.0751373 |
0.1401316 |
0.0004225 |
|
sp|P62750|RL23A_HUMAN |
60S ribosomal protein L23a |
0.6930985 |
0.0008642 |
1.189059 |
0.0170376 |
0.4874163 |
0.0005725 |
|
sp|P07737|PROF1_HUMAN |
Profilin-1 |
0.735016 |
0.0374251 |
1.066458 |
0.2291982 |
0.3612709 |
0.0009833 |
|
sp|P27635|RL10_HUMAN |
60S ribosomal protein L10 |
0.7687711 |
0.0078934 |
0.9977811 |
0.9774836 |
0.7807455 |
0.0424448 |
|
sp|P62244|RS15A_HUMAN |
40S ribosomal protein S15a |
0.6297469 |
0.0310408 |
1.2447751 |
0.2475055 |
0.3974015 |
0.006189 |
|
sp|P40261|NNMT_HUMAN |
Nicotinamide N-methyltransferase |
0.4948544 |
0.0145719 |
0.6104929 |
0.089145 |
0.4008726 |
0.0055721 |
|
sp|O75396|SC22B_HUMAN |
Vesicle-trafficking protein SEC22b |
0.8383446 |
0.0477434 |
0.9134793 |
0.2489925 |
0.6230699 |
0.0051087 |
|
sp|P63000|RAC1_HUMAN |
Ras-related C3 botulinum toxin substrate 1 |
0.7182943 |
0.0425175 |
0.9622406 |
0.6886851 |
0.4670397 |
0.0072975 |
|
sp|P52565|GDIR1_HUMAN |
Rho GDP-dissociation inhibitor 1 |
0.6346489 |
0.0200063 |
0.9114226 |
0.2442628 |
0.5560255 |
0.0041023 |
|
sp|Q02543|RL18A_HUMAN |
60S ribosomal protein L18a |
0.7989309 |
0.0054508 |
0.9987536 |
0.99314 |
0.5924663 |
0.001603 |
|
sp|P31949|S10AB_HUMAN |
Protein S100-A11 |
0.7316054 |
0.0105927 |
0.9486079 |
0.454312 |
0.5093769 |
0.0051478 |
|
sp|Q9NUQ6|SPS2L_HUMAN |
SPATS2-like protein |
0.8603883 |
0.0377549 |
0.8679588 |
0.4357617 |
0.6844587 |
0.0203066 |
|
sp|Q9UBY9|HSPB7_HUMAN |
Heat shock protein beta-7 |
0.5591881 |
0.0039141 |
0.8718413 |
0.1764828 |
0.1434866 |
0.0124455 |
|
sp|P62851|RS25_HUMAN |
40S ribosomal protein S25 |
0.7520575 |
0.0210642 |
1.257262 |
0.0216226 |
0.640693 |
0.0285225 |
|
sp|P60866|RS20_HUMAN |
40S ribosomal protein S20 |
0.5922481 |
0.0372282 |
1.135938 |
0.1952282 |
0.5971945 |
0.0331289 |
|
sp|Q15121|PEA15_HUMAN |
Astrocytic phosphoprotein PEA-15 |
0.7424016 |
0.0342224 |
0.8453032 |
0.2365656 |
0.5568212 |
0.01295 |
|
sp|Q07020|RL18_HUMAN |
60S ribosomal protein L18 |
0.7694229 |
0.0284774 |
0.9228408 |
0.3425165 |
0.3917271 |
0.0355194 |
|
sp|P10599|THIO_HUMAN |
Thioredoxin |
0.6320711 |
0.0364051 |
1.015246 |
0.849784 |
0.3958604 |
0.0290566 |
|
sp|P04080|CYTB_HUMAN |
Cystatin-B |
0.6261729 |
0.0164924 |
0.7203438 |
0.1116478 |
0.5600607 |
0.0261583 |
|
sp|Q99584|S10AD_HUMAN |
Protein S100-A13 |
0.6776413 |
0.0141411 |
0.936109 |
0.683051 |
0.4091621 |
0.0109403 |
|
sp|Q96FQ6|S10AG_HUMAN |
Protein S100-A16 |
0.7541828 |
0.0181902 |
0.7892096 |
0.1416664 |
0.4442775 |
0.0107498 |
|
sp|Q9UK76|HN1_HUMAN |
Hematological and neurological expressed 1 protein |
0.6930878 |
0.0069185 |
0.8315814 |
0.0583053 |
0.6368985 |
0.0217168 |
|
sp|Q9Y281|COF2_HUMAN |
Cofilin-2 |
0.7557867 |
0.0425518 |
0.79008 |
0.2477008 |
0.411954 |
0.0016992 |
|
sp|P07951|TPM2_HUMAN |
Tropomyosin beta chain |
0.2181936 |
0.0301534 |
0.3476089 |
0.1504305 |
0.1699484 |
0.0294093 |
|
Common Proteins in 12, 24 and GBCs |
|
sp|P21333|FLNA_HUMAN |
Filamin-A |
0.6491441 |
1.197E-20 |
0.775251 |
3.467E-08 |
0.2333875 |
0 |
|
sp|Q14315|FLNC_HUMAN |
Filamin-C |
0.691992 |
1.206E-23 |
0.8109596 |
5.152E-08 |
0.1362745 |
9.121E-29 |
|
sp|O75369|FLNB_HUMAN |
Filamin-B |
0.8066565 |
4.197E-13 |
0.8043511 |
4.036E-16 |
0.2220095 |
6.755E-27 |
|
sp|O43707|ACTN4_HUMAN |
Alpha-actinin-4 |
0.7782484 |
5.953E-08 |
0.825819 |
6.121E-06 |
0.5644021 |
7.637E-12 |
|
sp|P02452|CO1A1_HUMAN |
Collagen alpha-1(I) chain |
0.1710951 |
9.168E-08 |
0.1382745 |
1.707E-13 |
0.0871599 |
1.366E-13 |
|
sp|P08133|ANXA6_HUMAN |
Annexin A6 |
0.7843405 |
5.397E-05 |
0.8396946 |
8.225E-05 |
0.1753338 |
1.19E-12 |
|
sp|Q05682|CALD1_HUMAN |
Caldesmon |
0.6998289 |
1.765E-06 |
0.8143998 |
0.0014214 |
0.4336191 |
1.744E-09 |
|
sp|P07355|ANXA2_HUMAN |
Annexin A2 |
0.7777895 |
0.0138044 |
0.8567227 |
0.0063894 |
0.6411493 |
9.041E-05 |
|
sp|P12814|ACTN1_HUMAN |
Alpha-actinin-1 |
0.791617 |
0.0101087 |
0.7677427 |
0.0018612 |
0.1750646 |
2.284E-09 |
|
sp|P08123|CO1A2_HUMAN |
Collagen alpha-2 (I) chain |
0.2235407 |
4.04E-08 |
0.1855634 |
2.282E-12 |
0.1483753 |
3.537E-10 |
|
sp|P13797|PLST_HUMAN |
Plastin-3 |
0.6665052 |
6.952E-07 |
0.68303 |
0.0014106 |
0.155993 |
1.821E-11 |
|
sp|P04075|ALDOA_HUMAN |
Fructose-bisphosphate aldolase A |
0.7604178 |
0.0073406 |
0.8671005 |
0.0114424 |
0.7167717 |
0.0005067 |
|
sp|P00558|PGK1_HUMAN |
Phosphoglycerate kinase 1 |
0.5859095 |
0.0001153 |
0.6541614 |
8.688E-07 |
0.6226673 |
4.852E-07 |
|
sp|P08729|K2C7_HUMAN |
Keratin, type II cytoskeletal 7 |
0.3680029 |
3.277E-09 |
0.5083517 |
3.862E-10 |
0.0634308 |
4.792E-08 |
|
sp|P04083|ANXA1_HUMAN |
Annexin A1 |
0.7572839 |
0.0002047 |
0.8503217 |
0.0470738 |
0.550002 |
1.28E-07 |
|
sp|P07996|TSP1_HUMAN |
Thrombospondin-1 |
0.7980919 |
0.000262 |
0.553084 |
7.377E-09 |
0.2897949 |
1.053E-07 |
|
sp|P05783|K1C18_HUMAN |
Keratin, type I cytoskeletal 18 |
0.4164745 |
1.808E-06 |
0.4301328 |
6.1E-07 |
0.1056535 |
4.488E-08 |
|
sp|O75083|WDR1_HUMAN |
WD repeat-containing protein 1 |
0.6917547 |
4.643E-06 |
0.7336982 |
0.0007094 |
0.3800338 |
1.092E-10 |
|
sp|Q99715|COCA1_HUMAN |
Collagen alpha-1 (XII) chain |
0.3545905 |
1.41E-13 |
0.2877428 |
4.501E-18 |
0.2026522 |
6.706E-15 |
|
sp|Q96AC1|FERM2_HUMAN |
Fermitin family homolog 2 |
0.7950298 |
7.873E-06 |
0.8533728 |
0.0033112 |
0.2169133 |
1.367E-10 |
|
sp|Q15942|ZYX_HUMAN |
Zyxin |
0.7646767 |
0.001499 |
0.8185034 |
0.0001323 |
0.1502384 |
7.344E-10 |
|
sp|O43852|CALU_HUMAN |
Calumenin |
0.5774046 |
9.216E-06 |
0.8483972 |
0.0222098 |
0.4965094 |
0.0002066 |
|
sp|P13674|P4HA1_HUMAN |
Prolyl 4-hydroxylase subunit alpha-1 |
0.6946718 |
2.918E-06 |
0.6132792 |
1.034E-05 |
0.2874632 |
5.012E-08 |
|
sp|O15460|P4HA2_HUMAN |
Prolyl 4-hydroxylase subunit alpha-2 |
0.6696345 |
3.711E-07 |
0.5143549 |
9.348E-08 |
0.2669969 |
3.101E-10 |
|
sp|P42224|STAT1_HUMAN |
Signal transducer and activator of transcription 1-alpha/beta |
0.8499698 |
0.0053961 |
0.7854432 |
0.0005595 |
0.4379452 |
5.75E-10 |
|
sp|Q99536|VAT1_HUMAN |
Synaptic vesicle membrane protein VAT-1 homolog |
0.8316366 |
0.0180953 |
0.8146253 |
0.0168569 |
0.2068785 |
0.0002163 |
|
sp|Q9UHB6|LIMA1_HUMAN |
LIM domain and actin-binding protein 1 |
0.7435201 |
2.34E-05 |
0.7350705 |
0.0005448 |
0.3019533 |
1.856E-08 |
|
sp|Q14847|LASP1_HUMAN |
LIM and SH3 domain protein 1 |
0.6822608 |
0.0040097 |
0.8205906 |
0.0125213 |
0.3513746 |
1.928E-06 |
|
sp|Q9Y696|CLIC4_HUMAN |
Chloride intracellular channel protein 4 |
0.6635472 |
0.0298623 |
0.6565596 |
0.0294536 |
0.259223 |
2.416E-06 |
|
sp|P18669|PGAM1_HUMAN |
Phosphoglycerate mutase 1 |
0.6600341 |
0.0005784 |
0.7290849 |
0.000285 |
0.5737495 |
0.0001545 |
|
sp|Q16643|DREB_HUMAN |
Drebrin |
0.7486963 |
0.0013527 |
0.8171875 |
0.0228791 |
0.3356167 |
1.741E-07 |
|
sp|Q16222|UAP1_HUMAN |
UDP-N-acetylhexosamine pyrophosphorylase |
0.6732854 |
0.0001079 |
0.7852986 |
0.0005487 |
0.3712067 |
5.819E-07 |
|
sp|P02461|CO3A1_HUMAN |
Collagen alpha-1 (III) chain |
0.2201081 |
0.0004393 |
0.1852412 |
0.0001674 |
0.1235914 |
0.0007796 |
|
sp|O00469|PLOD2_HUMAN |
Procollagen-lysine,2-oxoglutarate 5-dioxygenase 2 |
0.3010921 |
1.377E-05 |
0.3480398 |
1.46E-07 |
0.1949575 |
4.065E-06 |
|
sp|P12109|CO6A1_HUMAN |
Collagen alpha-1 (VI) chain |
0.4696823 |
8.107E-05 |
0.4169624 |
1.04E-06 |
0.7281033 |
0.001178 |
|
sp|O00151|PDLI1_HUMAN |
PDZ and LIM domain protein 1 |
0.8575081 |
0.0064386 |
0.808583 |
0.0013904 |
0.7191794 |
0.0001499 |
|
sp|Q9BUF5|TBB6_HUMAN |
Tubulin beta-6 chain |
0.5307726 |
0.0061257 |
0.6090587 |
0.0079562 |
0.277919 |
0.0004736 |
|
sp|Q02818|NUCB1_HUMAN |
Nucleobindin-1 |
0.8226624 |
0.0371084 |
0.663159 |
1.352E-05 |
0.6716899 |
0.0183368 |
|
sp|O43399|TPD54_HUMAN |
Tumor protein D54 |
0.6686246 |
0.005498 |
0.7417749 |
0.011344 |
0.4392767 |
1.561E-05 |
|
sp|P07858|CATB_HUMAN |
Cathepsin B |
0.7033579 |
0.014844 |
0.6877466 |
0.0012351 |
0.2995372 |
0.0003164 |
|
sp|Q99439|CNN2_HUMAN |
Calponin-2 |
0.7122491 |
0.0170487 |
0.6706736 |
0.0230627 |
0.5064756 |
0.0001678 |
|
sp|Q02809|PLOD1_HUMAN |
Procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 |
0.8466675 |
0.0085338 |
0.8482934 |
0.002677 |
0.3619584 |
4.314E-05 |
|
sp|P17813|EGLN_HUMAN |
Endoglin |
0.5273556 |
0.004124 |
0.6475987 |
0.0055206 |
0.147541 |
0.0005594 |
|
sp|O15143|ARC1B_HUMAN |
Actin-related protein 2/3 complex subunit 1B |
0.697738 |
0.0270338 |
0.84576 |
0.0351022 |
0.2651682 |
0.0004373 |
|
sp|P12110|CO6A2_HUMAN |
Collagen alpha-2 (VI) chain |
0.6012068 |
0.0088132 |
0.3789701 |
0.0007036 |
0.3206771 |
0.0017214 |
|
sp|P21980|TGM2_HUMAN |
Protein-glutamine gamma-glutamyltransferase 2 |
0.5693054 |
4.331E-05 |
0.627192 |
0.0004874 |
0.1962446 |
0.0002049 |
|
sp|P26373|RL13_HUMAN |
60S ribosomal protein L13 |
0.6572507 |
0.0010244 |
0.7834665 |
0.0095265 |
0.6630557 |
0.0040335 |
|
sp|Q14192|FHL2_HUMAN |
Four and a half LIM domains protein 2 |
0.7846096 |
0.0029767 |
0.8173597 |
0.0012308 |
0.2725918 |
0.0390995 |
|
sp|Q93052|LPP_HUMAN |
Lipoma-preferred partner |
0.7990149 |
0.0225724 |
0.8337976 |
0.0406539 |
0.292731 |
0.0001339 |
|
sp|Q16647|PTGIS_HUMAN |
Prostacyclin synthase |
0.8080279 |
0.0319709 |
0.7324285 |
0.0019337 |
0.263861 |
0.0002228 |
|
sp|O15371|EIF3D_HUMAN |
Eukaryotic translation initiation factor 3 subunit D |
0.864748 |
0.0383892 |
0.8346376 |
0.0167422 |
0.6714211 |
0.003605 |
|
sp|P51911|CNN1_HUMAN |
Calponin-1 |
0.4608479 |
0.0003309 |
0.4437445 |
0.0019445 |
0.2158564 |
0.007823 |
|
sp|Q13642|FHL1_HUMAN |
Four and a half LIM domains protein 1 |
0.4609712 |
0.0001142 |
0.5427488 |
0.0001992 |
0.2829264 |
1.591E-06 |
|
sp|P20908|CO5A1_HUMAN |
Collagen alpha-1 (V) chain |
0.379388 |
0.0068348 |
0.3544788 |
0.0003237 |
0.2353524 |
0.0026677 |
|
sp|O60568|PLOD3_HUMAN |
Procollagen-lysine,2-oxoglutarate 5-dioxygenase 3 |
0.7638809 |
0.0008531 |
0.8055949 |
0.0047301 |
0.556466 |
0.0003241 |
|
sp|P26022|PTX3_HUMAN |
Pentraxin-related protein PTX3 |
0.416522 |
0.0040385 |
0.2322244 |
0.000583 |
0.186485 |
0.0139001 |
|
sp|Q9H425|CA198_HUMAN |
Uncharacterized protein C1orf198 |
0.631039 |
0.0133804 |
0.6294332 |
0.0089014 |
0.264752 |
0.0014479 |
|
sp|P09486|SPRC_HUMAN |
SPARC |
0.4096919 |
0.0060709 |
0.1639415 |
0.0023144 |
0.2305094 |
0.0028895 |
|
sp|P06756|ITAV_HUMAN |
Integrin alpha-V |
0.5613986 |
0.018194 |
0.6385075 |
0.0016505 |
0.482797 |
0.0014006 |
|
sp|O15173|PGRC2_HUMAN |
Membrane-associated progesterone receptor component 2 |
0.8195758 |
0.0447193 |
0.8143579 |
0.041582 |
0.3442066 |
0.0120293 |
|
sp|P05121|PAI1_HUMAN |
Plasminogen activator inhibitor 1 |
0.3748078 |
0.002411 |
0.2813975 |
0.0062054 |
0.1704524 |
0.0032371 |
|
sp|P11047|LAMC1_HUMAN |
Laminin subunit gamma-1 |
0.7204638 |
0.0263982 |
0.8115935 |
0.047751 |
0.5165971 |
0.0042495 |
|
sp|P41567|EIF1_HUMAN |
Eukaryotic translation initiation factor 1 |
0.5903546 |
0.0021622 |
0.5996968 |
0.0012622 |
0.8147202 |
0.0319759 |
|
sp|P05787|K2C8_HUMAN |
Keratin, type II cytoskeletal 8 |
0.7084652 |
0.0319926 |
0.6759541 |
0.0142614 |
0.3713869 |
0.0315686 |
|
sp|P08729|K2C7_HUMAN |
Keratin, type II cytoskeletal 7 |
0.3680029 |
3.277E-09 |
0.5083517 |
3.862E-10 |
0.0634308 |
4.792E-08 |
|
sp|P05787|K2C8_HUMAN |
Keratin, type II cytoskeletal 8 |
0.7084652 |
0.0319926 |
0.6759541 |
0.0142614 |
0.3713869 |
0.0315686 |

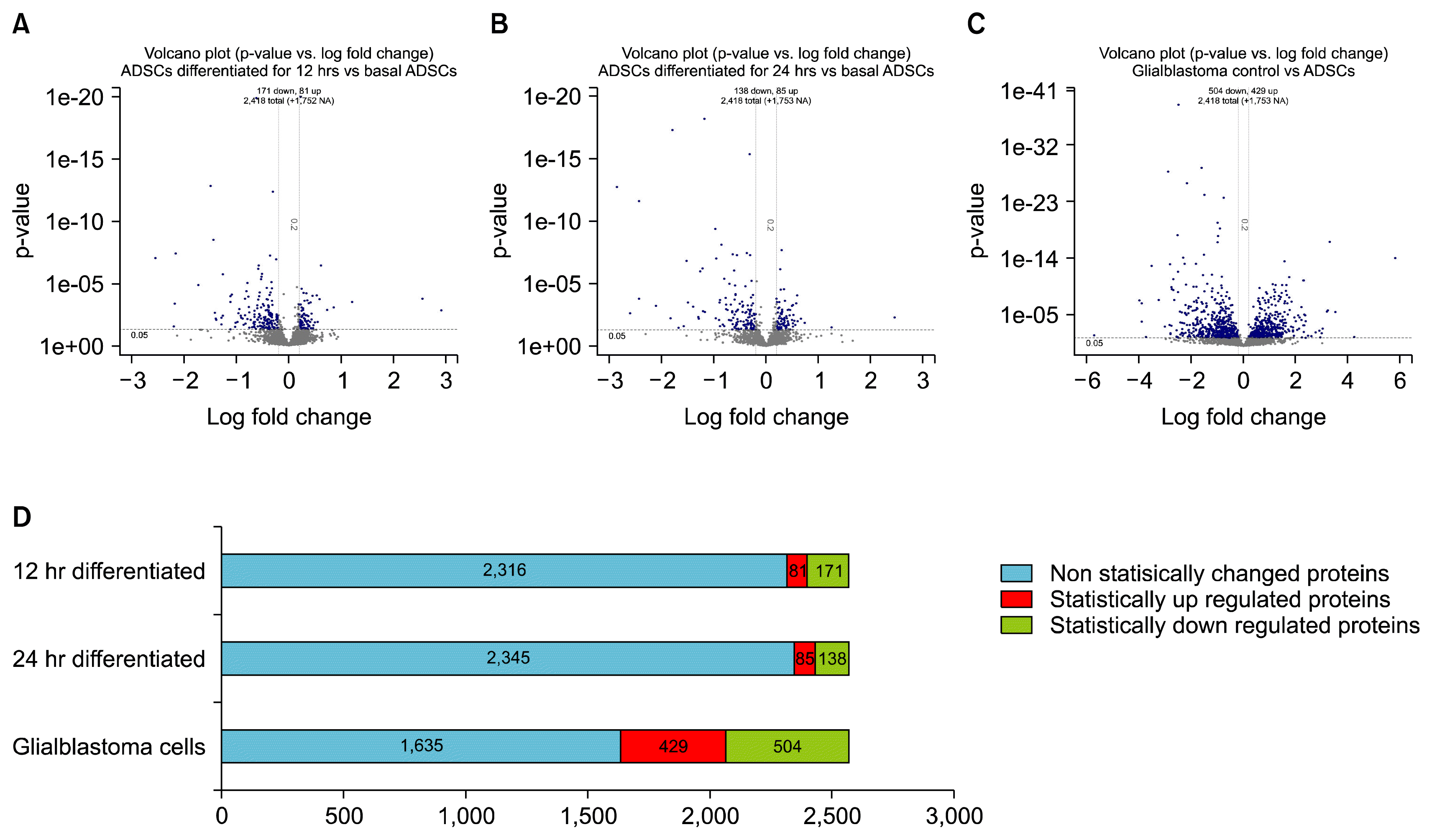
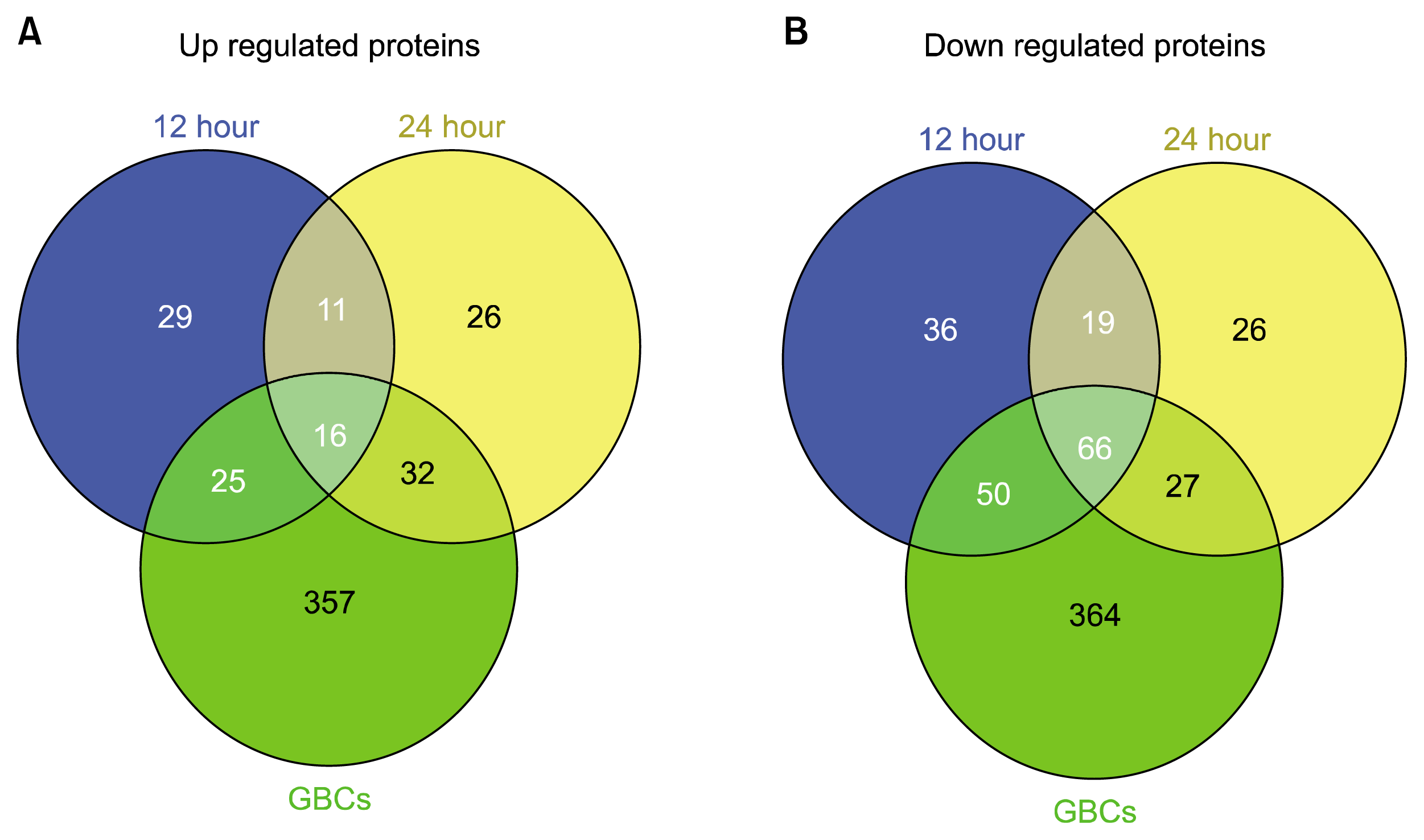
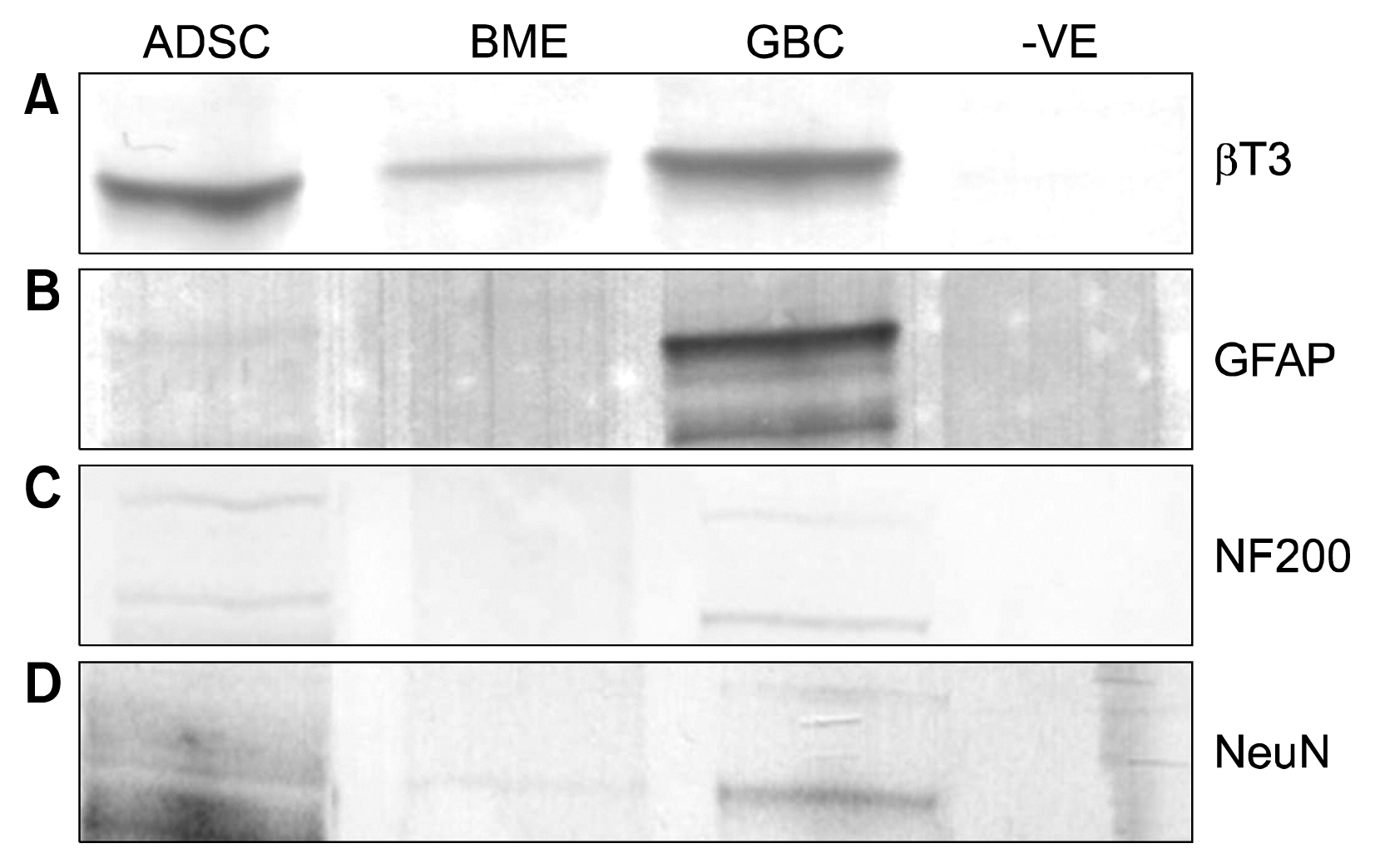
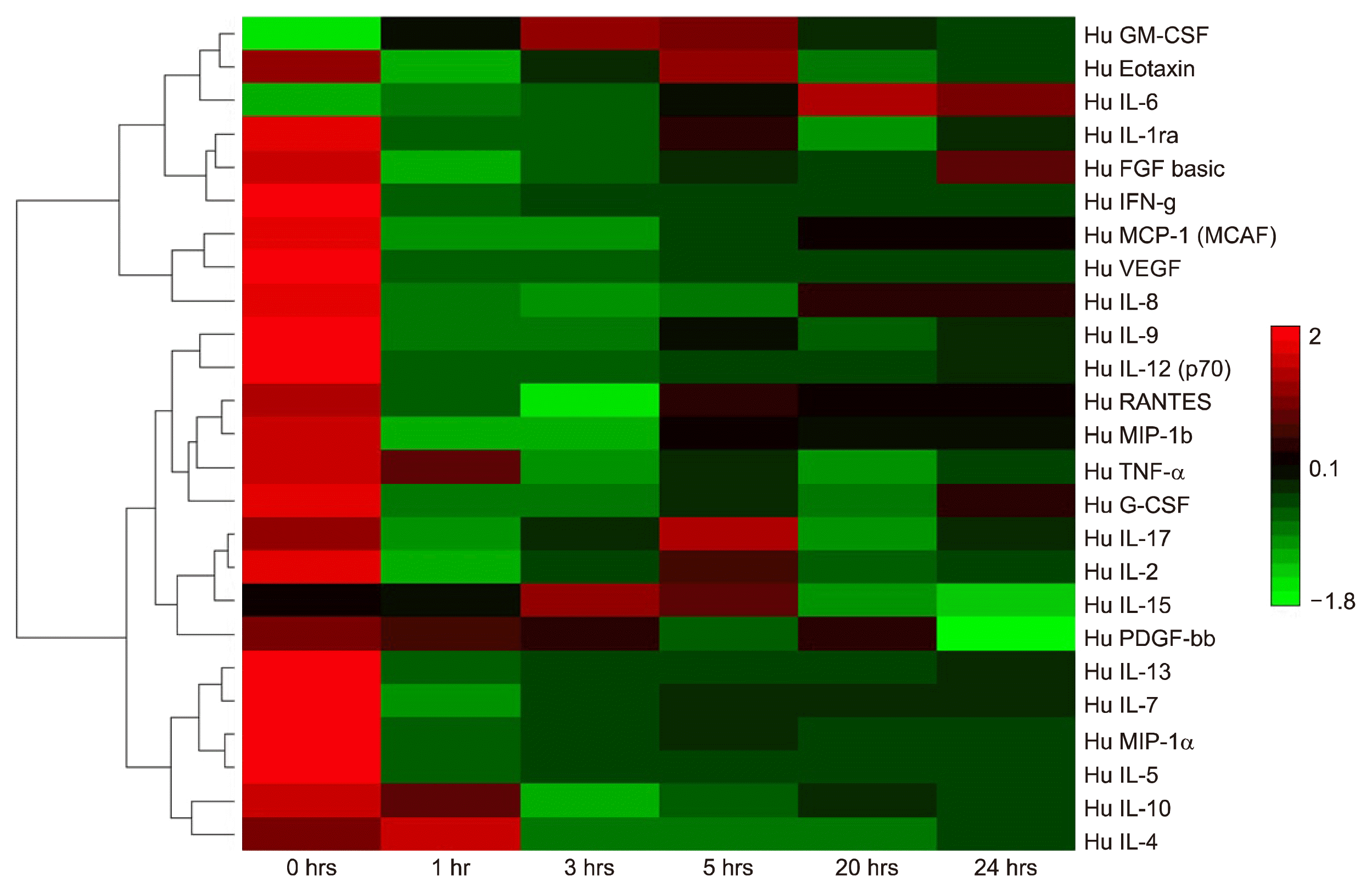




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download