Abstract
Objectives
To compare the effect of three different cryoprotectants on basic stem cell characteristics for the possibility of using well defined, dimethyl sulfoxide (DMSO) and serum free freezing solutions to cryopreserve human Wharton’s jelly-derived mesenchymal stem cells (WJMSCs) following controlled rate freezing protocol.
Methods
The mesenchymal stem cells isolated from human Wharton’s jelly were cryopreserved using 10% DMSO, 10% polyvinylpyrrolidone (PVP) and a cocktail solution comprising of 0.05 M glucose, 0.05 M sucrose and 1.5 M ethylene glycol following controlled rate freezing protocol. We investigated the post-thaw cell viability, morphology, proliferation capacity, basic stem cell characteristics, in vitro differentiation potential and apoptosis-related gene expression profile before and after cryopreservation.
Results
The cryoprotectant 10% DMSO has shown higher post-thaw cell viability of 81.2±0.58% whereas 10% PVP and cocktail solution have shown 62.87±0.35% and 72.2±0.23%, respectively at 0 h immediately thawing. The cell viability was further reduced in all the cryopreserved groups at 24 h later post-thaw culture. Further, the complete elimination of FBS in cryoprotectants has resulted in drastic reduction in cell viability. Cryopreservation did not alter the basic stem cell characteristics, plasticity and multipotency except proliferation rate. The expression of pro-apoptotic BAX and p53 genes were higher whilst p21 was lower in all the cryopreserved groups when compare to the control group of WJMSCs.
Conclusion
Although 10% DMSO has shown higher post-thaw cell viability compare to 10% PVP and cocktail solution, the present study indicates the feasibility of developing a well-defined DMSO free cryosolution which can improve storage and future broad range applications of WJMSCs in regenerative medicine without losing their basic stem cell characteristics.
Mesenchymal stem cells (MSCs) are non-hematopoietic adult stem cells which are first isolated and characterized from the bone marrow (1). These cells are both self-renewal and multipotent with their ability to differentiate into mesoderm, endoderm as well as ectoderm lineages (2–4). Although bone marrow and adipose tissue derived MSCs are considered to be the main source and hence are being extensively studied for cell therapy, it has also been reported that MSCs could occur in virtually all post-natal organs and tissues (5). MSCs of different adult tissue origin exhibit not only varying but also limited proliferative capacity, which makes them difficult to scale up for therapeutic applications (6). Further the cell procurement involves highly invasive procedure with relatively low cell numbers which necessitate further ex vivo expansion, and the cell numbers are also been found to decline with the donor age. However, the MSCs derived from fetal/peri-natal tissues such as placenta, umbilical cord Wharton’s jelly, amniotic fluid (7) possess high proliferation and differentiation capabilities. Furthermore, ease of isolation and scalability of these fetal tissue derived MSCs with significantly higher expression of pluripotency markers compared to those derived from adult tissues made them an alternative source for cell based therapies (8). These tissue specific exhibition of differential capability and functionality of MSCs could be a result of their occurrence in different extracellular milieu (9).
The human umbilical cord was first described and reported by Thomas Wharton in 1656 which is embryologically derived at day 26 of gestation (10) but the successive isolation of MSCs from wharton’s jelly portion of the umbilical cord was reported in 1991 (11). Wharton’s jelly (WJ) is the connective tissue which is gelatinous in nature present between the amniotic epithelium and the umbilical vessels providing protection within the umbilical cord (12). The MSCs presented in Wharton’s jelly are often regarded as umbilical cord matrix (UCM) cells and their occurrence is believed to be trapped during their migration from the aortic-gonadotropin-mesonephric region to the fetal liver through the umbilical cord in the early embryogenesis period (13), additionally due to their dependence on the source of oxygen and nutrients, they would most likely be located closest to the vasculature. The isolation, culture and characterization of WJMSCs have been already reported and many previous studies indicate their possible applications in the treatment of many diseases such as graft versus host disease (GVHD), cancer, systemic lupus erythematosus (SLE), liver and kidney injury.
Due to broad range applications of MSCs in regenerative medicine, their cryopreservation and long-term storage has become an absolute necessity. Although there are well developed cryopreservation protocols for hematopoietic stem cells, an efficient protocol for non-hematopoietic stem cells is still need to be optimized. However avoiding the lethal effect of cooling and thawing processes during cellular cryopreservation has currently becomes the greatest challenge and thereby developing a standard cryopreservation formulation and protocol for WJMSCs is indispensible, since they cannot be propagated for longer duration under standard in vitro culture conditions. The cooling rate and the use of cryoprotective agents (CPA) further play a major role on survivability of cells in any cryopreservation protocol. It has been shown that the maximum viability can be achieved for a wide variety of cells following controlled-rate freezing (14), this improved post-freeze cell viability could be explained by controlled ice nucleation and inhibition of release of latent heat of fusion (15). It has been reported that the vitrification (rapid cooling) of human umbilical cord mesenchymal stem cells has shown to be a reliable and effective method of cryopreservation by using high concentration of CPAs (16). However, slow freezing with reduced concentration of CPAs has gain much of interest in order to decrease the effect of osmotic shock and chemical toxicity exerted by CPAs (17). Among a wide variety of CPAs, dimethyl sulfoxide (DMSO) with its high membrane permeability and fetal bovine serum (FBS) being rich in growth factors and proteins are most commonly employed for cell cryopreservation. Using 10% (v/v) DMSO and up to 90% (v/v) FBS has become more common formulation to preserve various kinds of stem cells despite both exhibit a considerable disadvantages. To ameliorate this drawback, MSCs were cryopreserved using both DMSO and FBS free systems comprising of different polymers either alone or in combination with ethylene glycol (EG), 1,2-propylene glycol (PG), trehalose (T), sucrose and/or glucose. In contrast to DMSO which penetrates quickly into the cell, the high molecular weight polymers such as polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), polyethylene oxide (PEO), or polyvinyl alcohol (PVA) are non-penetrating and seems to exert their cryoprotection extracellularly when present at 10~40% concentrations (18). These high molecular weight agents tend to have increasingly high viscosities at low temperature and possibly prohibit water molecules to form ice crystals (19).
Thus, the present study aimed to investigate the effect of three different cryoprotectants on WJMSCs using controlled rate freezing protocol that would allow an efficient storage of WJMSCs without affecting their basic characteristics. Further, an attempt has been made to eliminate xenogenic FBS completely in the cryoprotectants. The efficacy of cryopreservation was evaluated by post-thaw viability, proliferation rate, surface and pluripotency marker expression, apoptosis and in vitro osteogenic and adipogenic differentiation ability of WJMSCs.
All chemicals and media were purchased from Sigma (St. Louis, MO, USA) and Gibco (Life Technologies, Burlington, ON, Canada) respectively, unless otherwise specified. Media employed for washing was Dulbecco’s phosphate buffered saline (DPBS) supplemented with 1 mg/ml poly-vinyl alcohol (PVA), 1% (v/v) penicillin-streptomycin (10,000 IU and 10,000 μg/ml, respectively, Pen-Strep). Advanced Dulbecco’s modified Eagle’s medium (ADMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 2 mM glutamine and 1% (v/v) Pen-Strep was used for cell culture. The pH and osmolality of all media were adjusted to 7.2 and 285±5 mOsm/l, respectively.
Human umbilical cords (n=5) from both sexes were obtained from full-term births (caesarean section or normal vaginal delivery) after obtaining the informed consent under approved medical guidelines set by the GNUH IRB-2012-09-004. Isolation of the Wharton’s jelly derived MSCs was carried out as previously described (20) with minor modifications. Briefly, the cord was cut into 2~3 cm lengths, rinsed several times with DPBS containing 1% (v/v) Pen-Strep. After being removed umbilical cord vessels, the mesenchymal tissue was minced into small pieces using fine scissors followed by two times wash with DPBS. Tissue was then digested with DPBS containing 1 mg/ml collagenase type I at 37°C for 40 min with gentle agitation. The digested tissue was sequentially passed through 100 μm and 40 μm nylon cell strainers (BD Falcon, MA, USA) in order to obtain a single cell suspension after enzyme being inactivated by adding ADMEM containing 30% (v/v) FBS. The isolated cells after being centrifuged at 500×g for 5 min were reconstituted and cultured in ADMEM supplemented with 10% (v/v) FBS at 37°C in a humidified atmosphere of 5% CO2 in air by changing the culture medium for every 3 days. When the cells became confluent, they were trypsinized using 0.25% (w/v) trypsin-ethylenediaminetetraacetic acid (EDTA) solution and centrifuged, and cell pellets were then harvested for further expansion or cryopreservation studies. Freshly isolated WJMSCs without undergoing the procedure of cryopreservation were treated as control. In the current study WJMSCs at passage 3 were used in all the experimentation under different treatment groups. Morphology of WJMSCs was analyzed under a light microscope. Images were taken at 100× magnification with Nikon DIAPHOT 300, Japan.
The CPAs were prepared according to the previously published literatures with minor modifications as and where specified. Solution A: 10% (v/v) PVP with an average molecular weight of 40,000 was prepared in ADMEM containing 10% (v/v) FBS at room temperature and the solution was then stored at 4°C overnight to obtain a homogeneous preparation (21), Solution B: cocktail solution was prepared in ADMEM containing 10% (v/v) FBS by dissolving 0.05 M glucose, 0.05 M sucrose and 1.5 M ethylene glycol (22), Solution C: 10% (v/v) DMSO solution was diluted with ADMEM supplemented with 10% (v/v) FBS. Solutions D, E and F were similar to Solutions A, B and C respectively but devoid of 10% (v/v) FBS.
After dissociation with 0.25% (w/v) trypsin-EDTA solution, WJMSCs were washed twice with ADMEM supplemented with 10% (v/v) FBS by centrifugation at 500×g for 5 min. Cell density was adjusted to 1×106 cells/ml using respective CPAs and transferred to 1.8 ml cryovials (Thermoscientific, Roskilde, Denmark) in 1 mL aliquots. The cryovials were then cooled at a pre-set freezing rate in a programmable controlled-rate freezer (Kryo 360, Planer Ltd, Middlesex, UK). The cells were equilibrated for 30 min at 1°C, then cooled following the programmed protocol in order: −2°C/min to −9°C, then −9°C to −9.1°C and held for 5 min; then −0.3°C/min to −40°C; then −10°C/min to −140°C (22). Then the cryovials were immediately plunged into liquid nitrogen (LN2) and stored in LN2 for three month.
For further analysis, the cryopreserved WJMSCs were thawed by immersing in a circulating water bath at 37°C for 1 min and were washed twice with ADMEM supplemented with 10% (v/v) FBS and 1% (v/v) Pen-Strep by centrifugation at 500×g for 5 min in order to remove CPAs.
Viability of WJMSCs was determined by trypan blue exclusion test. WJMSCs were stained with 0.2% trypan blue solution immediately after thawing (0 h) and 24 h later post-thaw culture. The numbers of dead and viable cells were recorded based on the development of blue colour by observing under a light microscope using hemocytometer. The percentage of viability of WJMSCs was calculated using the formula: total number of viable cells/total number of cells ×100.
The proliferative capacity of WJMSCs was evaluated by population doubling time (PDT). Briefly, WJMSCs from all the experimental groups were plated at 2×103 cells in each well of the 24-well culture plate in triplicate. Culture was maintained up to 14 days and the cell number was recorded for every 2 days interval. PDT of WJMSCs was calculated using a formula, PDT=t (log2)/(logNt− logN0), where t represents the culture time, and N0 and Nt are the initial and final WJMSC numbers before and after seeding, respectively.
Evaluation of DNA content and cell surface antigens of WJMSCs was done by using flow cytometer (BD FACS Calibur; Becton Dickinson, NJ, USA) in triplicates. DNA content of WJMSCs was evaluated by fixing a total of 1×106 cells/ml in 70% ethanol at 4°C for 4 h. The cells were then washed twice with DPBS and stained with 10 μg/ml propidium iodide solution for 15 min. DNA content of each cell was measured and categorized as G0/G1, S or G2/M phase of the cell cycle. Phenotyping of cell surface antigens of WJMSCs was carried out by labelling cells (1×105 per marker) with fluorescein isothiocyanate-conjugated CD34 (BD Pharmingen, CA, USA, FITC Mouse Anti-Human CD34), CD45 (Santa Cruz Biotechnology, FITC Mouse Anti-Human CD45), CD90 (BD Pharmingen, FITC Mouse Anti-Human CD90) and unconjugated CD73 (Santa Cruz Biotechnologies, Mouse monoclonal) and CD105 (Santa Cruz Biotechnologies, Mouse monoclonal IgG2a) for 30 min. Unconjugated primary antibodies were treated with FITC-conjugated goat anti-mouse IgG (BD Pharmingen) for 30 min in dark. Mouse IgG1 served as isotype matched negative control (BD Pharmingen). A total of 10,000 labeled cells per sample were acquired and counted in a Becton Dickinson FACS Calibur flowcytometer (FACS, Becton Dickinson, NJ, USA) and the results were analyzed by cell Quest Prosoftware (Becton Dickinson).
WJMSCs from both fresh and cryopreserved groups were assessed for their in vitro differentiation ability into adipogenic and osteogenic lineages as per the previously published protocols (23), by culturing in a lineage specific medium under suitable condition for 21 days in triplicates. Adipogenic medium was comprised of 1 μM dexamethasone, 10 μM insulin, 100 μM indomethacin, and 500 μM isobutylmethylxanthine (IBMX). Osteogenic medium was comprised of 0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate, and 10 mM glycerol-2-phosphate. Adipogenesis was confirmed by the accumulation of lipid droplets by staining with Oil red O solution whereas Osteogenesis by Alizarin red and von Kossa staining.
WJMSCs in all the experimental groups were grown until 80% confluence in the culture medium and the expression of transcriptional factors, apoptosis-related genes and lineage-specific markers were analysed by RT-PCR in triplicates. Total RNA was isolated using RNeasy mini kit (Qiagen, CA, USA). A total of 2 μg RNA was used to synthesize complementary DNA (cDNA) using an Omni-script RT kit (Qiagen), 10 μM oligodT primer (Invitrogen, CA, USA) at 37°C for 60 min. Real time PCR was carried out using Rotor gene Q (Qiagen), using Rotor Gene SYBR green PCR kit (Qiagen). A total of 50 ng cDNA was added to 12.5 μl SYBR green mix, 5.5 μl RNase free water and 1 μl each of forward and reverse primers at 1 pM (final volume 25 μl). The assay was performed with initial denaturation at 95°C for 10 min, followed by 40 PCR cycles at 95°C for 10 s, 60°C for 6 s and 72°C for 4 s, followed by a melting curve from 60°C to 95°C at 1°C/s, and then cooling at 40°C for 30 s, according to the manufacturer’s protocol. CT values and melting curves of each sample were analysed using Rotor-Gene Q Series Software (Qiagen). The amplified products were evaluated by electrophoresis on 1.5% agarose gel and images were analyzed using zoom browser EX5.7 software (Canon). Reference gene YWHAZ (Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide) was used as the housekeeping gene for normalization of the data. The relative level of target gene expression was calculated according to 2−ΔΔCT method (24). The primers used are listed in Table 1.
Protein lysate of all the samples was prepared using RIPA buffer (Thermoscientific) containing protease inhibitors. Protein concentration was determined by Microplate BCA Protein Assay kit (Thermo Scientific), a total of 25 μg each protein sample was separated using 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE, Mini Protean, BioRad) and transferred onto polyvinylidene difluoride membranes (PVDF, Millipore, USA). Membranes were then incubated with primary antibodies of goat anti-Oct-3/4 (43–50 kDa, 1:200, Santa Cruz), rabbit anti-Sox-2 (34 kDa, 1:200, Santa Cruz), goat anti-Nanog (35 kDa, 1:200, Santa Cruz), rabbit anti-Bax (22 kDa, 1:1000, Enzo, NY, USA), rabbit anti-Bcl-2 (28 kDa, 1:1000, Cell Signalling, MA, USA), mouse anti-p53 (53 kDa, 1:200, Santa Cruz), rabbit anti-p21 (21 kDa, 1:200, Santa Cruz) and rabbit anti-β actin (45 kDa, 1:1000, Cell Signalling) for overnight at 4°C followed by incubation with horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG (1:10000, Santa Cruz), goat anti-rabbit IgG (1:10000, Santa Cruz) and goat anti-mouse IgG (1:10000, Santa Cruz) secondary antibodies for 1 h at room temperature. Immunoreactivity was detected by enhanced chemiluminescence (ECL; Supersignal, West Pico Chemiluminescent substrate, PIERCE, IL, USA) and exposed to x-ray films.
The statistical differences between experimental groups were analyzed by one-way ANOVA using SPSS 21.0 followed by Tukey’s multiple comparisons test. Data were presented as mean±standard error of the estimate of mean value (S.E.M.) of at least three separate experiments. In each experiment data were taken in triplicate. Differences among groups were considered significant at p<0.05, and were denoted by different superscript letters.
After 3 days of culture, colonies of adherent and fibroblastic spindle-like cell morphology were observed in all the groups (Fig. 1). The percentage viability of WJMSCs cryopreserved with different cryoprotectants was assessed immediately post thawing (0 h) and 24 h later. The results suggest that there was a significant reduction (p<0.05) of viability in all the groups followed by cryopreservation with their respective CPAs compare to the control group. At 0 h post thawing (Fig. 2A), Solution C has shown higher viability efficiency of 81.2±0.58% whereas Solution A being the minimum of 62.87±0.35% against the control group (97.83±0.32%). However, in the present study the complete elimination of FBS in the cryosolution has further reduced the viability efficiency of all the cryoprotectants used. The viability was drastically reduced to 6.8±0.23% when 10% (v/v) PVP was used solely with the complete elimination of FBS in the cryosolution (Solution D). At 24 h post-thaw culture (Fig. 2B), Solution B has shown significantly (p<0.05) reduced viability compare to Solution A and Solution C (Based on the initial cell numbers at 0 h). On the other hand, all the cryoprotectants with complete elimination of FBS have followed the similar trend with further reduction in their cryoprotection efficiency as observed immediately after thawing (0 h). Based on these observations, only cryoprotectants supplemented with 10% FBS were chosen for subsequent experiments.
Evaluation of PDT showed that the proliferative capacity of WJMSCs was significantly (p<0.05) reduced after cryopreservation with all the three cryoprotectants (Fig. 3A). Doubling time was found to be 54.28±0.05 h, 55.13±0.03 h, 55.79±0.12 h, 55.49±0.06 h for control, Solution A, Solution B, and Solution C respectively. There was a significant (p<0.05) difference found in all the phases of cell cycle among different cryopreserved groups in comparison to the control as analyzed by FACS (Fig. 3B), and the proportion of cells in S phase of the cell cycle was 23.26±0.17%, 21.01±0.16%, 14.68±0.23% and 19.59±0.14%, whereas in G0/G1 phase was 66.62±0.12%, 76.55±0.15%, 82.73±0.18% and 78.25±0.37%, and those in the G2/M phase was 10.12±0.06%, 2.44±0.31%, 2.60±0.15% and 2.16±0.49% in control, Solution A, Solution B and Solution C respectively.
There was no significant difference found in the expression of cell surface antigens between cryopreserved and control groups (Fig. 4). Cultured WJMSCs under both control and cryopreserved groups were constitutively expressed the transcription factors OCT4, SOX2 and NANOG indicating their undifferentiated state as revealed by RT-PCR and western blot analysis both at m-RNA and protein level respectively (Fig. 6B & 6E).
Both control and cryopreserved WJMSCs upon in vitro differentiation under specific conditions using specific differentiation medium were able to differentiate into mesenchymal lineages (Adipogenic & osteogenic), indicating their plasticity and multipotency. Adipogenic differentiation was confirmed after 21 days by visualization of the accumulation of cytoplasmic lipid droplets (Stained red) seen with Oil Red O staining (Fig. 5A). Cells were also expressed adipocyte specific markers such as peroxisome proliferative activated receptor gamma (PPARγ), fatty acid binding protein 4 (FABP4) and lipoprotein lipase (LPL) (Fig. 7B). The formation of calcium deposits upon osteogenic induction was demonstrated by Alizarin red and von Kossa staining (Fig. 5B). Further, the differentiated cells expressed osteocyte specific markers such as runt-related transcription factor-2 (Runx2), osteonectin and bone morphogenetic protein 2 (BMP2) (Fig. 7D).
Total RNA and protein was isolated from WJMSCs at passage 3 under both control and cryopreserved groups. Real-time PCR was performed to determine the expression level of transcription- and apoptosis-related genes. Results indicate that although the expression level of transcription factors such as OCT-4, SOX2 and Nanog in cryopreserved groups were slightly higher than a control group, but it did not statistically significant (Fig. 6A). The expression level of pro-apoptotic genes such as BAX and p53 was found to be higher in all the cryopreserved groups with significantly (p<0.05) reduced expression of p21 in comparison to the control group. Moreover, WJMSCs cryopreserved with Solution B has shown significantly (p<0.05) higher mRNA level of BAX and p53 compare to other cryopreserved groups. Conversely the expression level of anti-apoptotic gene BCL2 was found to be reduced when compare to the control group with no observed statistical differences (Fig. 6C). The BAX/BCL2 ratio were found to be 1.31±0.08%, 4.12±0.44% and 1.67±0.13% in WJMSCs cryopreserved using Solution A, Solution B and Solution C respectively. Similar results were also observed at protein level in the western blot for both transcription- and apoptosis-related proteins (Fig. 6E). The expression level of adipogenic specific markers such as PPARγ, FABP4 and LPL were significantly (p<0.05) increased to 2.2~4.9 folds upon in vitro adipogenic induction in all the experimental groups. Further, there was no statistically significant difference found in PPARγ and LPL expression among both control and cryopreserved groups but the level of FABP in WJMSCs cryopreserved using Solution C was significantly (p<0.05) reduced compare to that in the control group (Fig. 7A). Similarly, upon osteogenic induction, the lineage specific markers such as RUNX2, Osteonectin and BMP2 were also found to be increased their expression level up to 1.4~4.7 folds (Fig. 7C).
In the current study, six different cryosolutions with varying composition of CPAs were used to cryopreserve WJMSCs following controlled rate freezing protocol and compared their cryopreservation efficiency. In the process of human cell cryopreservation or tissue banking, the DMSO and serum are being extensively used and there is a growing concern about their effect on cryopreserved cells/tissue and post transplantation complications (25). Further, the use of DMSO can change cellular characteristics and alter genetic and/or epigenetic properties (26). These drawbacks made researchers to search for alternative cryoprotectants comprising of less harmful components with higher cryoprotection efficiency. Thereby, the main purpose of this study was to develop an alternative cryoprotectant to conventional DMSO that can efficiently be used in the controlled rate freezing of WJMSCs and also to eliminate FBS completely in the cryosolution to avoid the xenogenic effects.
The long term viability of any biospecimen is thought to be depending on the cooling rate in any cryopreservation protocol. During freezing, the cooling rate also affects both rate and size of intracellular and/or extracellular ice crystal formation. An elevated intracellular ice formation is more frequently associated with rapid cooling, as water does not have time to migrate out of the cell, whereas cell can lose maximum water with reduced intracellular ice formation under slow cooling rate. However, other factors such as latent heat of fusion and type of nucleating agents used do variably affect the cryoprotection of different CPAs. Therefore, the present study has mainly focused on using the controlled rate freezing to preserve WJMSCs based on the recent findings in the previously published protocol (22).
The chemical components such as polyvinylpyrrolidone, ethylene glycol, glucose, sucrose, FBS and DMSO were used in the cryosolutions under the current investigation. We found that conventional DMSO had higher cryoprotection efficiency when compare to other two cryosolutions used. Cryopreservation of WJMSCs with 10% (v/v) DMSO and 10% (v/v) FBS in ADMEM (Solution C) has demonstrated a cell viability of 81.2±0.52% immediately post-thawing at 0 h, this is in agreement with a previous report where human bone marrow-derived MSCs frozen with DMSO have shown 83.8±2.9% post-thaw cell viability (27). The cell viability in Solution B comprising of 0.05 M glucose, 0.05 M sucrose, 1.5 M ethylene glycol and 10% (v/v) FBS was found to be lesser than previous report (22), where they have demonstrated that around 79% of viability can be achieved using similar cryoprotectant upon dental tissue cryopreservation. This variability could be a reason of different cell types used and also the type of cryopreservation (Tissue or cell). Thus the current finding envisages that Solution B could more likely be used for tissue storage rather than cells. However additional studies are needed to conclude this bias in cryoprotection towards tissue or cell preservation. On the other hand cell viability obtained using Solution A with 10% (v/v) PVP and 10% (v/v) FBS in ADMEM is comparable with the previously published literature, which reports around 69.2±6.7% viability could be achieved upon cryopreservation of adipose tissue-derived adult stem cells when 10% (v/v) PVP along with 10% (v/v) FCS has been used as a cryoprotectant (21). However, assessing the cell viability immediately after thawing cannot be a true measure of representing the efficacy of cryopreservation. A further reduction in cell viability was observed with all the cryosolutions after 24 h post-thaw culture, where Solution B has shown significantly (p<0.05) reduced viability compare to Solution A and Solution C. This has shown better cryoprotection than Solution A when assessed immediately after thawing (0 h). This reduction in cell viability of post-thaw culture could be related to apoptotic and necrotic processes which are known to occur within 24 h and are not apparently considered immediately after thawing (28). Serum is a complex mixture and probably contains various constituents such as cytokines and growth factors, and is often used in combination with other cryoprotectants albeit its mechanism of cryoprotection remains unclear. Being xenogenic, its application in cultivation or cryopreservation of cells used for clinical purposes has directly linked to the detection of anti-FBS antibodies in the recipient (29). Nevertheless, FBS is known to possess inherent variation between sources and batches. Therefore, complete elimination or an alternative to FBS is indispensible and is the subject of current research. The present study aimed to eliminate FBS completely in the cryosolutions. However, the cell viability was significantly (p<0.05) reduced as noted both immediately at 0 h and 24 h later post-thaw culture. Moreover in the absence of FBS, cell viability (0 h) was drastically reduced to 6.80±0.23% in Solution D comprising of 10% (v/v) PVP in ADMEM compared to that in the presence of FBS and also compared to other two cryosolutions. Therefore in the present study it is observed that neither Solution A nor Solution B or Solution C were able to provide an efficient cryoprotection when used alone as a single cryoprotectant in the absence of FBS. Similar result was also reported for the cryopreservation of human embryonic stem cells where the absence of FBS caused a poor survival rate (30). These findings indicate that adding FBS remarkably increase the cryopreservation efficiency of cryoprotectants by yet unknown factors. It is unclear that whether some of the components in FBS can act as stabilizers for CPAs during cryopreservation, especially for external cryoprotectants such as PVP as observed in the current study or the presence of these additives may possibly be result in either the formation of long strands of polymer or pushes polymer chains close to each other so that an effective encapsulation of the cell could occur when polymers are used as cryoprotectants. However, the present study could not address this correlation and hence further investigations are needed to unravel the mechanism behind this synergistic effect. Furthermore, based on the results obtained in the present study, it seems that certain amount of permeating CPA is required to increase the efficacy of cryoprotectants in protecting cells from freezing damages. An improved cell viability was observed when permeating CPA ethylene glycol was used in Solution B along with non-permeating glucose and sucrose in the cryosolution in comparison to Solution A where only non-permeating PVP was used. However, this cannot simply be explained since the phase change of the extracellular medium in different cryosolutions during any freezing experiments could also results in variable cell viability as cells experience different supercooling rates.
There was no notable difference found between control and cryopreserved groups in terms of morphology, pluri-potent and surface markers expression. Morphology of cultured WJMSCs has shown fibroblast-like cells capable of adhesion and proliferation on plastic dish surface. WJMSCs in the present study displayed a doubling time of approximately 54 h. whereas it has been reported to be approximately 40 h in the previous study (31), this variability could be a result of different culture and calculation method followed for cell proliferation assays. The expression of early transcription factors such as OCT4, SOX2 and NANOG are generally used to confirm the presence of adult stem cells (32). In the present study, the cryopreservation has not changed the expression of early transcription factors both at mRNA and protein level in WJMSCs. Therefore cells retain their characteristics upon cryopreservation with all the cryoprotectants used, similar results were also reported by a previous report (33).
We have analyzed the expression of cell surface markers of WJMSCs before and after cryopreservation. CD73, CD90 and CD105 are known to be expressed by WJMSCs (34). Flow cytometric data revealed that WJMSCs in both control and cryopreserved groups were positive for CD73, CD90 and CD105 while negative for the hematopoietic marker CD34 and CD45. The results obtained were similar in all the groups with no statistically significant differences. Thus, the immunophenotypic characteristics of WJMSCs were not affected by cryopreservation and the results obtained are in concordance with the previously published data (35). Nevertheless, these findings suggest that even after cryopreservation cells preserved their adhesion/cell to cell contact property, as many of these surface proteins are speculated to be involved in cell-cell and cell-matrix interactions (18). In the present study, thawed WJMSCs retain their capacity to differentiate towards adipocytes and osteocytes similar to those in the control group and hence the present cryopreservation protocol did not affect their plasticity as well as the multipotency.
During cryopreservation, cells have to struggle to cope up with extrinsic damaging agents (CPAs). Certain CPAs can cause an inevitable accumulation of damage that leads to the deterioration of cell components; thereby cell death could results from both necrosis and apoptosis. However, it has been reported that due to cryoinjury, apoptosis rather than cellular necrosis could occur after slow freezing of human embryonic stem cells (hES) which results in reduced cell viability. Further this reduction was prominent during incubation at 37°C over 90 min period (36). Apoptosis can be initiated by two major pathways intrinsic/mitochondrial and extrinsic. Intrinsic pathway is tightly regulated by the BCL-2 family of proteins, which is further classified into 3 groups: (i) anti-apoptotic multidomain members (BCL-2, BCL-XL and Mcl-1), which consists of four BCL-2 homology domains (BH1, BH2, BH3 and BH4), (ii) pro-apoptotic multidomain members (Bax and Bak), lacking the BH4 domain and (iii) pro-apoptotic BH3 only proteins (Bid, Bim and Bad). In contrast, the death receptors from the TNF (tumor necrosis factor) receptor family that include Fas/CD95 and the TRAIL (TNF-related apoptosis-inducing ligand) receptors can initiate an extrinsic pathway at the plasma membrane. In the current investigation, we have studied the expression of apoptotic related genes both at mRNA and protein level and found that apoptosis could be the reason in the loss of WJMSCs post-thaw viability. This was further supported by a significant reduction in post-thaw cell proliferation capacity, which indicates how cryopreservation affects cell functions. RT-PCR and western blot analysis revealed that the WJMSCs under all the cryopreserved groups were found to be associated with apoptosis. Particularly the cells cryopreserved using Solution B comprising of ethylene glycol, glucose, sucrose and 10% (v/v) FBS in ADMEM has shown higher expression of proapototic genes such as BAX and p53 indicating a possible occurrence of DNA damage, which was also indicated by the cell cycle analysis based on the fact that apoptosis is also regulated by genes that are involved in the cell cycle progression. Present study demonstrated that the percentage of cells in G0/G1 phase was higher in cells cryopreserved in Solution B compared to that in control and other cryopreserved groups. It was also noted that these percentage of cells at G0/G1 phase are directly proportional to the expression level of p53 in all the groups studied. This prolonged arrest of cells at this phase is more likely an index of activated DNA repair mechanism before progression into the next phase of cell cycle. p53 is a nuclear DNA-binding phosphoprotein exists normally as a tetramer and is able to bind to specific DNA sequences. A variety of stimuli can activate p53 either by increasing its half-life or translational initiation rate of its mRNA (37) and influences cell proliferation by acting predominately in the G1 phase of the cell cycle progression. The activated p53 can induce the expression of p21 which in turn inhibits cyclin D/Cdks eventually leads to an arrest of cell cycle at G1. Interestingly, in our study the expression of p21 was reduced in all the cryopreserved groups indicating that p53 can also regulate Cdks independent of p21. Earlier it has been reported that p53 can also downregulates Cdk2 through the regulation of CAK activity (38). The results obtained in the present study also indicates a direct involvement of p53 in arresting cell cycle possibly through interacting with CAK complex without the need of Cdk inhibitors. BCL2 gene encodes a protein that acts as an anti-apoptotic and blocks programmed cell death without affecting cellular proliferation (39) whereas BAX protein, a member of the BCL-2 family promotes apoptosis (40). Thereby the ratio of BAX to BCL2 indicates the susceptibility of a cell to apoptosis (40). In the current study, there was a strong increase in BAX/BCL2 ratio indicating an occurrence of apoptosis upon cryopreservation. However, the probable mode of apoptosis cannot be explained, as other downstream proteins of BCL2 family were not studied in the current investigation and the present study strongly endorses that these proteins should not be overlooked in the future experiments.
In conclusion, the conventional 10% DMSO supplemented with 10% FBS has shown higher post-thaw cell viability when compare to other two cryoprotectants investigated. Nevertheless, the present study also demonstrated that, though there was a possible occurrence of apoptosis upon cryopreservation with all the cryoprotectants as revealed by the gene expression profile, the WJMSCs were found to be preserved their basic stem cell characteristics. This indicates the possibility of developing a well-defined DMSO free cryosolution for WJMSCs cryopreservation. The post-thaw cell viability shows that FBS plays a major role during cryopreservation and it cannot be eliminated completely. Hence, the present study suggests that this could possibly be replaced by the development of other alternative complex protein sources. The present study also indicated the significance of the presence of certain amount of permeating CPA in the cryosolution to increase the cryoprotection, which shows an option to develop cocktail cryosolutions. However, the present study has tried to evaluate the effect of three different cryoprotectants on WJMSCs by using single controlled rate freezing protocol and, hence, further research is needed to assess the effect of other freezing rates to achieve an efficient storage of WJMSCs followed by in vivo studies to evaluate their post-transplantation complications.
Acknowledgments
This work was supported by the Korean Health Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (grant number HI13C1596), the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (NRF-2014R1A1A2058807) and Gyeongsang National University Research Foundation Grant (GNUHBIF-2014-0006).
References
1. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976; 4:267–274. PMID: 976387.
2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.

3. Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004; 40:1275–1284. DOI: 10.1002/hep.20469. PMID: 15562440.

4. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000; 61:364–370. DOI: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. PMID: 10931522.

5. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.

6. Abdulrazzak H, Moschidou D, Jones G, Guillot PV. Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface. 2010; 7(Suppl 6):S689–706. DOI: 10.1098/rsif.2010.0347.focus. PMID: 20739312. PMCID: 2988276.

7. Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010; 2:81–92. DOI: 10.4252/wjsc.v2.i4.81.

8. Sabapathy V, Sundaram B, VMS , Mankuzhy P, Kumar S. Human Wharton’s Jelly Mesenchymal Stem Cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014; 9:e93726. DOI: 10.1371/journal.pone.0093726.

9. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12. DOI: 10.1186/1478-811X-9-12. PMID: 21569606. PMCID: 3117820.

10. Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005; 23:220–229. DOI: 10.1634/stemcells.2004-0166. PMID: 15671145.

11. McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991; 19:29S. DOI: 10.1042/bst019029s.

12. Taghizadeh RR, Cetrulo KJ, Cetrulo CL. Wharton’s Jelly stem cells: future clinical applications. Placenta. 2011; 32(Suppl 4):S311–S315. DOI: 10.1016/j.placenta.2011.06.010.

13. Wang XY, Lan Y, He WY, Zhang L, Yao HY, Hou CM, Tong Y, Liu YL, Yang G, Liu XD, Yang X, Liu B, Mao N. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood. 2008; 111:2436–2443. DOI: 10.1182/blood-2007-07-099333.

14. Brockbank KGM, Covault JC, Taylor MJ. Cryopreservation manual: a guide to cryopreservation techniques. Mariette, USA: Thermo Farma Sci. Publ. Gr;2001.
15. Pegg DE. Principles of cryopreservation. Day JG, Stacey G, editors. Cryopreservation and freeze-drying protocols. 2nd ed. New Jersey: Humana Press Inc;2007. p. 39–74. DOI: 10.1007/978-1-59745-362-2_3.

16. Massood E, Maryam K, Parvin S, Mojgan M, Noureddin NM. Vitrification of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells. Cryo Letters. 2013; 34:471–480.
17. Luciano AM, Chigioni S, Lodde V, Franciosi F, Luvoni GC, Modina SC. Effect of different cryopreservation protocols on cytoskeleton and gap junction mediated communication integrity in feline germinal vesicle stage oocytes. Cryobiology. 2009; 59:90–95. DOI: 10.1016/j.cryobiol.2009.05.002. PMID: 19460364.

18. Balci D, Can A. The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr Stem Cell Res Ther. 2013; 8:60–72. DOI: 10.2174/1574888X11308010008.

19. Fuller BJ. Cryoprotectants: the essential antifreezes to protect life in the frozen state. Cryo Letters. 2004; 25:375–388.
20. Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008; 3:e1451. DOI: 10.1371/journal.pone.0001451.

21. Thirumala S, Wu X, Gimble JM, Devireddy RV. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods. 2010; 16:783–792. DOI: 10.1089/ten.tec.2009.0552.

22. Park BW, Jang SJ, Byun JH, Kang YH, Choi MJ, Park WU, Lee WJ, Rho GJ. Cryopreservation of human dental follicle tissue for use as a resource of autologous mesenchymal stem cells. J Tissue Eng Regen Med. 2014; DOI: 10.1002/term.1945. [Epub ahead of print]. PMID: 25052907.

23. Subbarao RB, Ullah I, Kim EJ, Jang SJ, Lee WJ, Jeon RH, Kang D, Lee SL, Park BW, Rho GJ. Characterization and evaluation of neuronal trans-differentiation with electrophysiological properties of mesenchymal stem cells isolated from porcine endometrium. Int J Mol Sci. 2015; 16:10934–10951. DOI: 10.3390/ijms160510934. PMID: 26006231. PMCID: 4463684.

24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001; 25:402–408. DOI: 10.1006/meth.2001.1262.

25. Ruiz-Delgado GJ, Mancías-Guerra C, Tamez-Gómez EL, Rodríguez-Romo LN, López-Otero A, Hernández-Arizpe A, Gómez-Almaguer D, Ruiz-Argüelles GJ. Dimethyl sulf-oxide-induced toxicity in cord blood stem cell transplantation: report of three cases and review of the literature. Acta Haematol. 2009; 122:1–5. DOI: 10.1159/000227267.

26. Ock SA, Rho GJ. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cell Transplant. 2011; 20:1231–1239. DOI: 10.3727/096368910X552835. PMID: 21294964.

27. Liu Y, Xu X, Ma X, Martin-Rendon E, Watt S, Cui Z. Cryopreservation of human bone marrow-derived mesenchymal stem cells with reduced dimethylsulfoxide and well-defined freezing solutions. Biotechnol Prog. 2010; 26:1635–1643. DOI: 10.1002/btpr.464. PMID: 20572296.

28. Heng BC. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2009; 41:376–380. DOI: 10.1016/j.tice.2009.01.004. PMID: 19261317.

29. Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007; 92:1208–1215. DOI: 10.3324/haematol.11446. PMID: 17666368.

30. Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, Kim SH, Moon SY. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod. 2005; 20:1779–1785. DOI: 10.1093/humrep/deh854. PMID: 15760949.

31. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014; 34:695–704. PMID: 24970492. PMCID: 4121354.

32. Kuleshova LL, Tan FC, Magalhães R, Gouk SS, Lee KH, Dawe GS. Effective cryopreservation of neural stem or progenitor cells without serum or proteins by vitrification. Cell Transplant. 2009; 18:135–144. DOI: 10.3727/096368909788341298. PMID: 19499702.

33. Naaldijk Y, Fedorova V, Stolzing A. Cryopreservation of human umbilical cord-derived mesenchymal stem cells in complex sugar based cryoprotective solutions. J Biotechnol Lett. 2013; 4:95–99.
34. Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010; 19:117–130. DOI: 10.1089/scd.2009.0177.

35. Roy S, Arora S, Kumari P, Ta M. A simple and serum-free protocol for cryopreservation of human umbilical cord as source of Wharton’s jelly mesenchymal stem cells. Cryobiolog. 2014; 68:467–472. DOI: 10.1016/j.cryobiol.2014.03.010.

36. Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ge Z, Ouyang HW, Lee EH, Cao T. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006; 13:433–445. DOI: 10.1007/s11373-005-9051-9.

37. Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia. 2000; 2:291–299. DOI: 10.1038/sj.neo.7900101. PMID: 11005563. PMCID: 1550296.

38. Schneider E, Montenarh M, Wagner P. Regulation of CAK kinase activity by p53. Oncogene. 1998; 17:2733–2741. DOI: 10.1038/sj.onc.1202504. PMID: 9840937.

39. Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990; 348:334–336. DOI: 10.1038/348334a0. PMID: 2250705.

40. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993; 74:609–619. DOI: 10.1016/0092-8674(93)90509-O. PMID: 8358790.

Fig. 1
Adherent, fibroblast-like morphology of WJMSCs from passage 3 on day 3 culture. Where (A)=Control, (B)=Solution A, (C)=Solution B and (D)=Solution C. Scale bar=100 μm.

Fig. 2
Viability percentage of WJMSCs under Control, Solution A, Solution B, Solution C, Solution D, Solution E and Solution F groups assessed immediately after thawing at 0 h (A), and at 24 h later post-thaw culture (based on the cell numbers obtained at 0 h) (B). Significant difference among groups was considered when p<0.05 and represented by different superscripts (lower case letters).
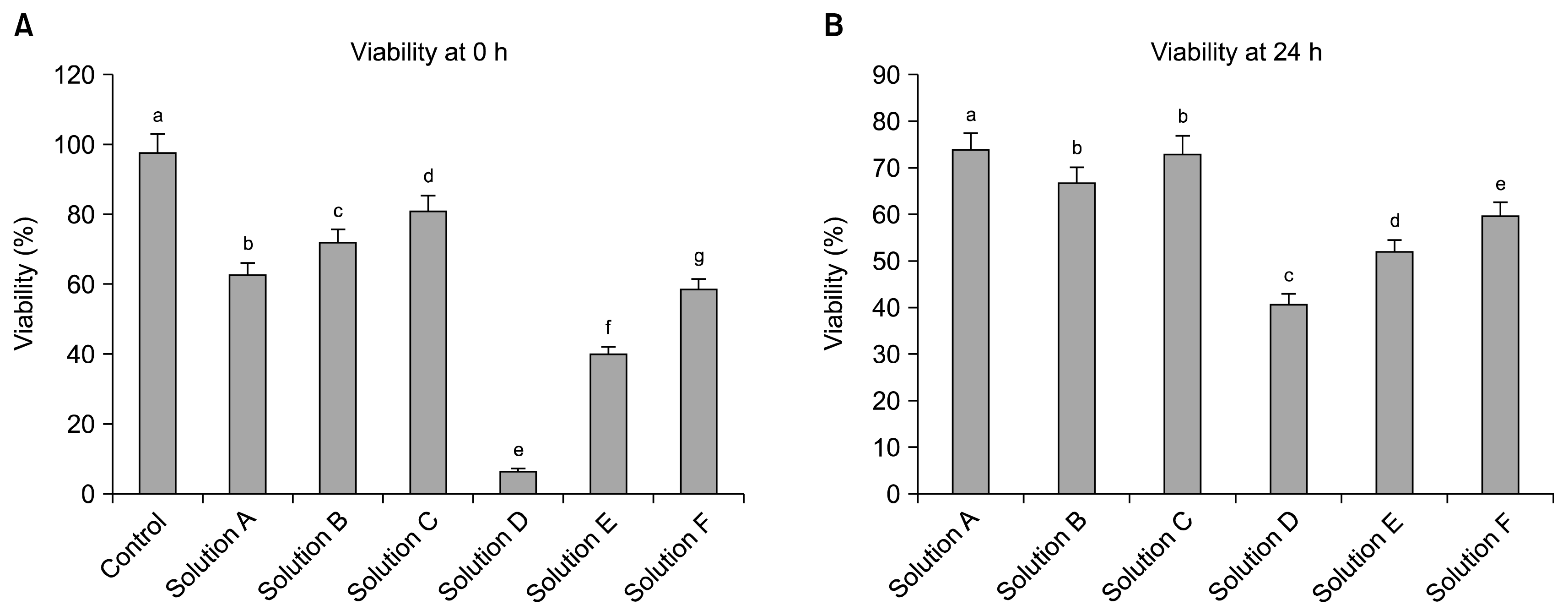
Fig. 3
Growth curves for WJMSCs under Control, Solution A, Solution B and Solution C over 14 days of in vitro culture. Cells were cultured in 24-well plates at an initial rate of 2×103 cells/well (A) and Flowcytometric analysis of cell cycle for WJMSCs under different groups. A total of 10,000 cells were counted for each sample in triplicates and the values are expressed as percentage mean±standard error of mean (SEM). Significant difference among groups was considered when p<0.05 and represented by different superscripts (lower case letters) (B).
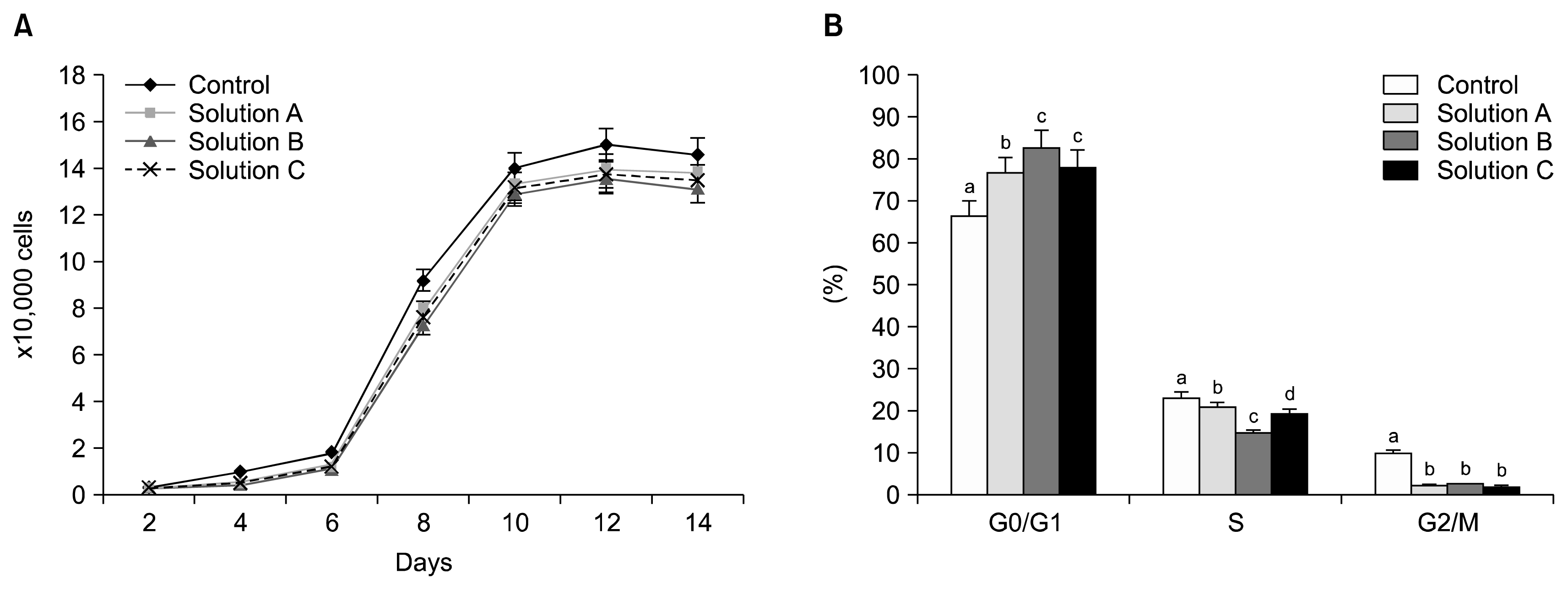
Fig. 4
Flowcytometric analysis of the expression of surface markers by WJMSCs under Control, Solution A, Solution B and Solution C groups. WJMSCs were negative for CD34 and CD45 whereas positive for CD73, CD90 and CD105 expression (percentage of expression is also indicated in the figure).
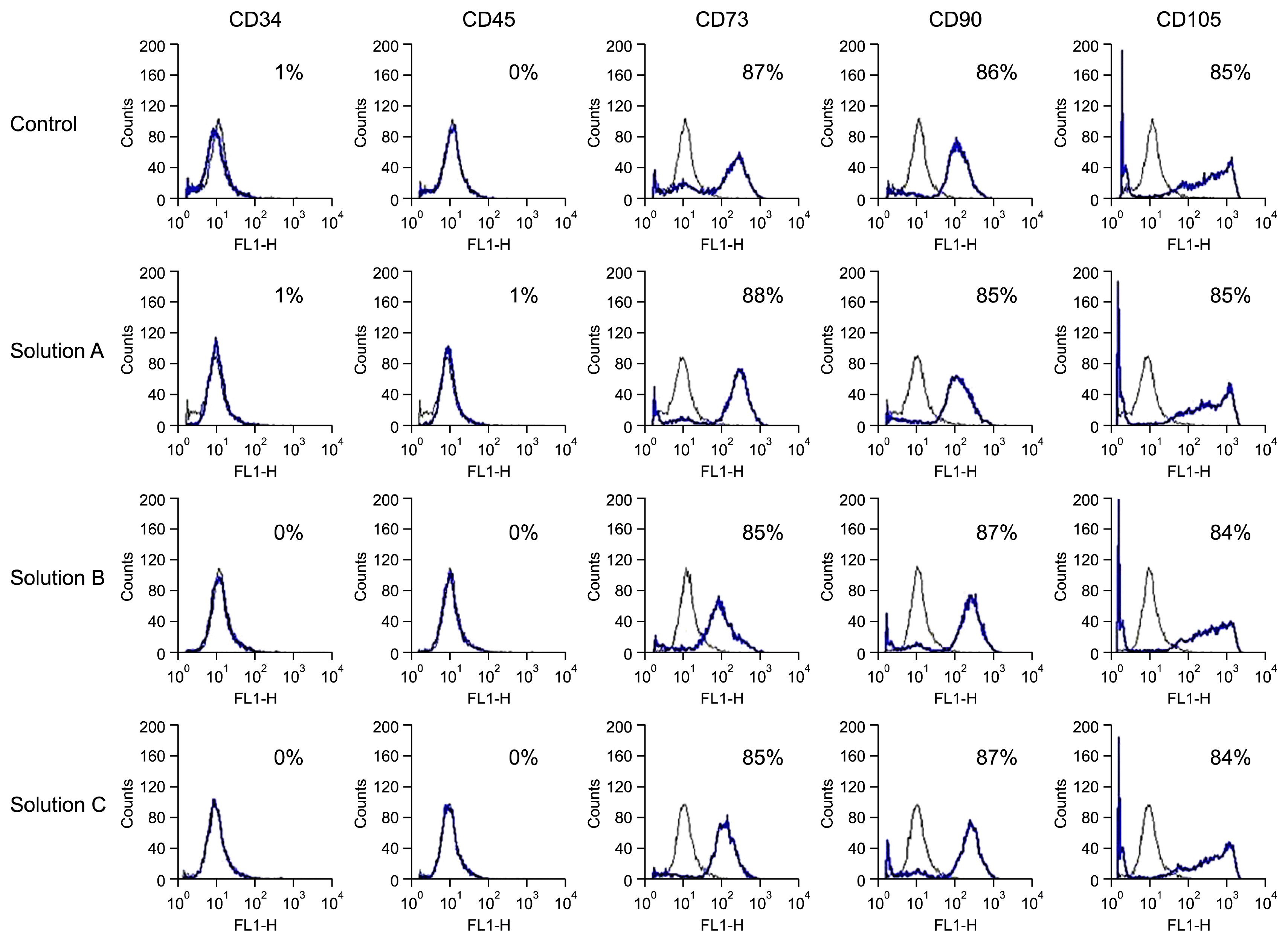
Fig. 5
In vitro differentiation potential of WJMSCs of Control, Solution A, Solution B and Solution C groups. WJMSCs were stained both before and after adipo/osteo lineage induction. Adipogenesis was indicated by Oil red O staining of lipid globules after induction (A). Confirmation of osteogenesis was done by Alizarin red and Von Kossa staining (B).
Fig. 6
RT-PCR and western blot analysis. Relative mRNA level of transcription factors such as OCT4, SOX2 and NANOG (A) and their product size (B). Relative mRNA level of apoptosis-related BAX, BCL2, p53 and p21 genes (C) and their product size (D). Different superscripts (lower case letters) represent significant difference (p<0.05) among groups. Western blot analysis of transcription- & apoptosis related proteins (E).
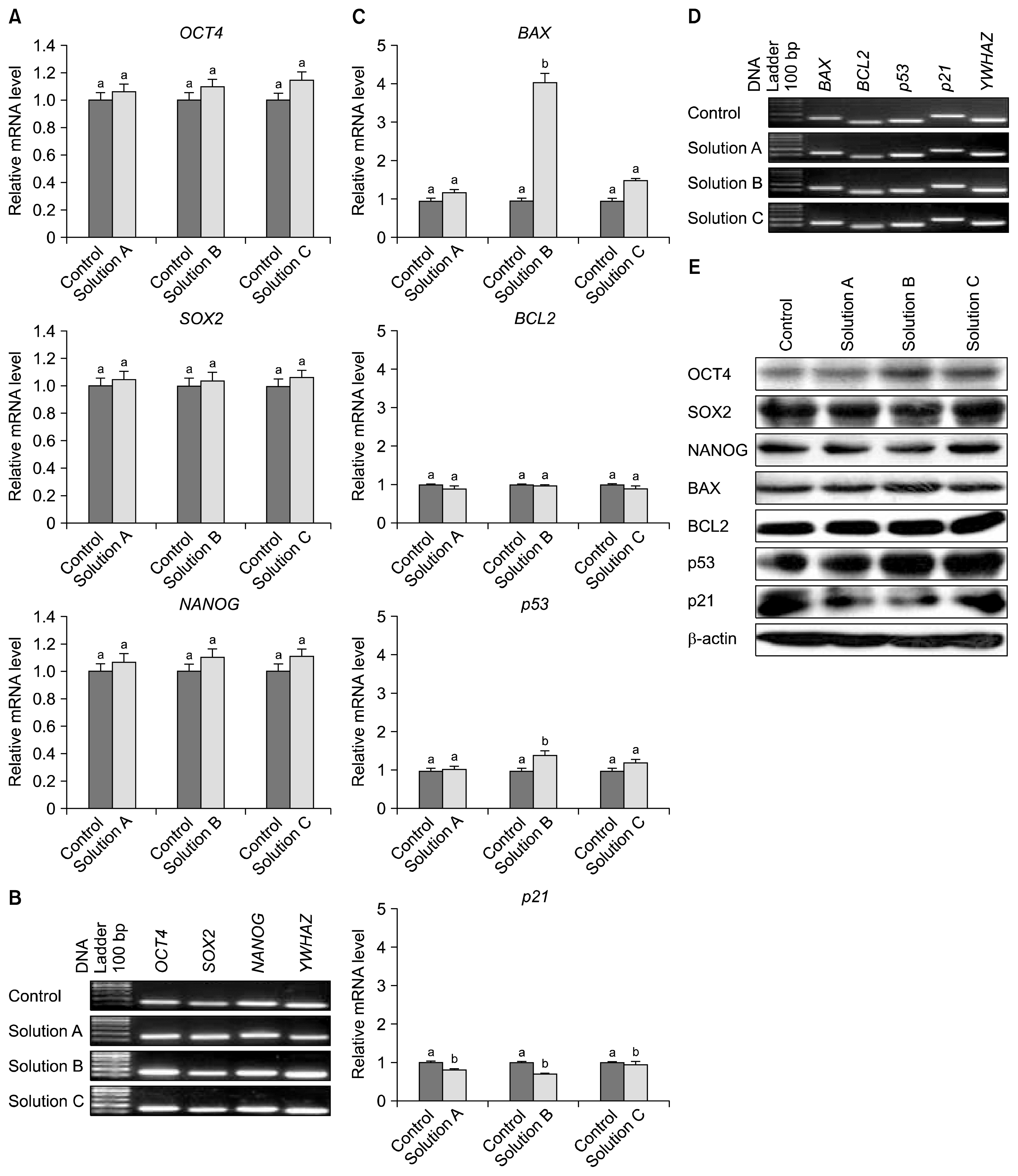
Fig. 7
RT-PCR analyses on adipogenesis and osteogenesis. Relative mRNA level of adipogenic specific markers (A) and their product size (B). Relative mRNA level of osteogenic specific markers (C) and their product size on agarose gel (D). Significant differences were considered when p<0.05 and represented by different superscripts (lower case letters).
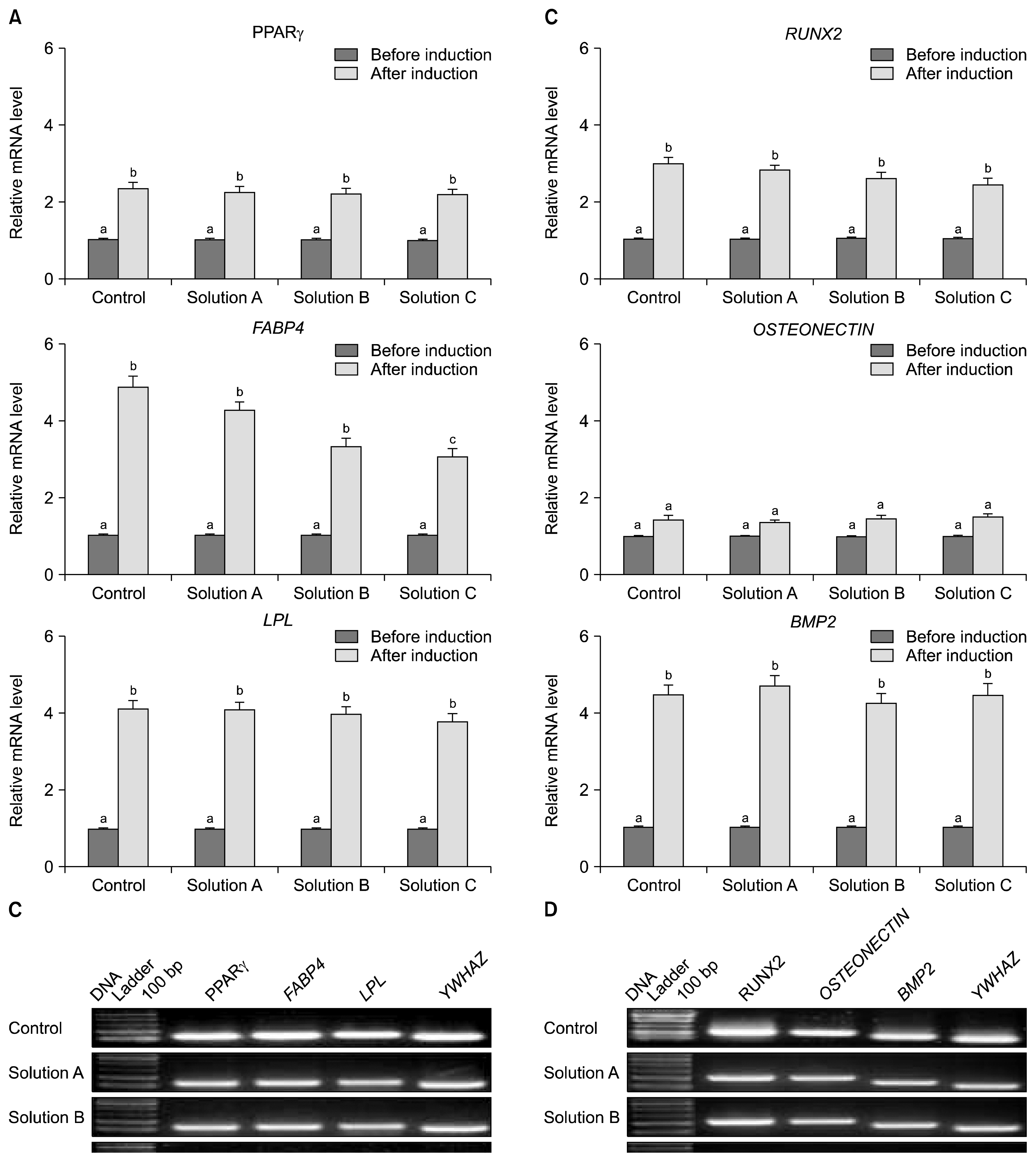
Table 1
List of primers used for the evaluation of transcription factors, apoptosis-related genes and lineage-specific markers in cultured WJMSCs by RT-PCR




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download