1. Meyer KC. Diagnosis and management of interstitial lung disease. Transl Respir Med. 2014; 2:1–13. DOI:
10.1186/2213-0802-2-4.

2. Garg J, Agrawal N, Marballi A, Agrawal S, Rawat N, Sule S, Lehrman SG. Amiodarone induced pulmonary toxicity: An unusual response to steroids. Am J Case Rep. 2012; 13:62–65. DOI:
10.12659/AJCR.882757. PMID:
23569490. PMCID:
3615935.

3. Mankikian J, Favelle O, Guillon A, Guilleminault L, Cormier B, Jonville-Béra AP, Perrotin D, Diot P, Marchand-Adam S. Initial characteristics and outcome of hospitalized patients with amiodarone pulmonary toxicity. Respir Med. 2014; 108:638–646. DOI:
10.1016/j.rmed.2014.01.014. PMID:
24565600.

4. Minder CM, Blaha MJ, Horne A, Michos ED, Kaul S, Blumenthal RS. Evidence-based use of statins for primary prevention of cardiovascular disease. Am J Med. 2012; 125:440–446. DOI:
10.1016/j.amjmed.2011.11.013. PMID:
22387091.

5. Malekinejad H, Mehrabi M, Khoramjouy M, Rezaei-Golmisheh A. Antifibrotic effect of atorvastatin on paraquat-induced pulmonary fibrosis: role of PPARγ receptors. Eur J Pharmacol. 2013; 720:294–302. DOI:
10.1016/j.ejphar.2013.10.013. PMID:
24161914.

6. Labusca L, Zugun-Eloae F, Nacu V, Mashayekhi K. Adipose derived stem cells for musculoskeletal regeneration: recent patents and future perspectives. Regen Med. 2013; 3:132–147.

7. Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, Hernandez-Ontiveros D, Kim DW, Metcalf C, Staples M, Dailey T, Vasconcellos J, Franyuti G, Gould L, Patel N, Cooper D, Kaneko Y, Borlongan CV, Bickford PC. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci. 2014; 34:313–326. DOI:
10.1523/JNEUROSCI.2425-13.2014. PMID:
24381292. PMCID:
3866490.

8. Zhu B, Ma AQ, Yang L, Dang XM. Atorvastatin attenuates bleomycin-induced pulmonary fibrosis via suppressing iNOS expression and the CTGF (CCN2)/ERK signaling pathway. Int J Mol Sci. 2013; 14:24476–24491. DOI:
10.3390/ijms141224476. PMID:
24351828. PMCID:
3876122.

9. Madkour NK, Ahmed M. Amelioration of amiodarone-induced lung fibrosis in rats by grape seed extract. J Appl Sci Res. 2013; 9:3698–3707.
10. Gnanasegaran N, Govindasamy V, Musa S, Kasim NH. Different isolation methods alter the gene expression profiling of adipose derived stem cells. Int J Med Sci. 2014; 11:391–403. DOI:
10.7150/ijms.7697. PMID:
24669199. PMCID:
3964446.

11. Ma T, Gong K, Ao Q, Yan Y, Song B, Huang H, Zhang X, Gong Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013; 22(Suppl 1):S113–S126. DOI:
10.3727/096368913X672181.

12. Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007; 7:26–34. DOI:
10.1186/1472-6750-7-26. PMID:
17537254. PMCID:
1904213.

13. Wei Y, Gong K, Zheng Z, Wang A, Ao Q, Gong Y, Zhang X. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J Mater Sci Mater Med. 2011; 22:1947–1964. DOI:
10.1007/s10856-011-4370-z. PMID:
21656031.

14. Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003; 107:2290–2293. DOI:
10.1161/01.CIR.0000070931.62772.4E. PMID:
12732608.

16. Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol. 2010; 298:L243–L251. DOI:
10.1152/ajplung.00242.2009.

17. Kiernan JK. Histological and histochemical methods. Theory and practice. 3rd ed. London, New York, New Delhy: Arnold Publisher;2001. p. 111–162.
18. Suvarna KS, Layton C, Bancroft JD. Pigments and minerals. Bancroft’s theory and practice of histological techniques. 7th ed. Oxford, UK, China: Churchill Livingstone Elsevier;2013. p. 241–242.
19. Ellis R. Perl’s prussian blue stain protocol. South Australia: Pathology Division, Queen Elizabeth Hospital;2007.
20. Sun W, Su Q, Cao X, Shang B, Chen A, Yin H, Liu B. High expression of polo-like kinase 1 is associated with early development of hepatocellular carcinoma. Int J Genomics. 2014; 2014:312130. DOI:
10.1155/2014/312130. PMID:
25019081. PMCID:
4074973.

21. Li L, Xia Y. Study of adipose tissue-derived mesenchymal stem cells transplantation for rats with dilated cardiomyopathy. Ann Thorac Cardiovasc Surg. 2014; 20:398–406. DOI:
10.5761/atcs.oa.13-00104. PMID:
24492176.

22. Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014; 370:1820–1828. DOI:
10.1056/NEJMra1204664. PMID:
24806161.

25. Valcheva-Kuzmanova S, Stavreva G, Dancheva V, Terziev L, Atanasova M, Stoyanova A, Dimitrova A, Shopova V. Effect of Aronia melanocarpa fruit juice on amiodarone-induced pneumotoxicity in rats. Pharmacogn Mag. 2014; 10:132–140. DOI:
10.4103/0973-1296.131024. PMID:
24914278. PMCID:
4048559.

26. Lee TH, Sottile J, Chiang HY. Collagen inhibitory peptide R1R2 mediates vascular remodeling by decreasing inflammation and smooth muscle cell activation. PLoS One. 2015; 10:e0117356. DOI:
10.1371/journal.pone.0117356. PMID:
25675397. PMCID:
4326127.

27. Gumuser G, Vural K, Varol T, Parlak Y, Tuglu I, Topal G, Sayit E. Assessment of lung toxicity caused by bleomycin and amiodarone by Tc-99m HMPAO lung scintigraphy in rats. Ann Nucl Med. 2013; 27:592–599. DOI:
10.1007/s12149-013-0722-8. PMID:
23558450. PMCID:
3742950.

28. Selman M, Vicens V, Mendoza C, Montaño M, Ramos C, Romero Y, Molina M. Subsets of fibroblasts show resistance to apoptosis independently of their interstitial lung disease origin. FASEB J. 2013; 27:1166.4.

29. Lee J, Hong EM, Koh DH, Choi MH, Jang HJ, Kae SH, Choi HS. HMG-CoA reductase inhibitors (statins) activate expression of PPARalpha/PPARgamma and ABCA1 in cultured gallbladder epithelial cells. Dig Dis Sci. 2010; 55:292–299. DOI:
10.1007/s10620-009-0734-3.

30. Khodayar MJ, Kiani M, Hemmati AA, Rezaie A, Zerafatfard MR, Rashidi Nooshabadi MR, Goudarzi M. The preventive effect of atorvastatin on paraquat-induced pulmonary fibrosis in the rats. Adv Pharm Bull. 2014; 4:345–349. PMID:
25436189. PMCID:
4137423.
31. Zhang Y, Dai L, Wu S, Chen P, Zhao S. Atorvastatin attenuates involvement of RhoA/Rho-kinase pathway and NF-κB activation in hypoxic pulmonary hypertensive rats. Chin Med J (Engl). 2014; 127:869–872.
32. Chen ZY, Liu SN, Li CN, Sun SJ, Liu Q, Lei L, Gao LH, Shen ZF. Atorvastatin helps preserve pancreatic β cell function in obese C57BL/6 J mice and the effect is related to increased pancreas proliferation and amelioration of endoplasmic-reticulum stress. Lipids Health Dis. 2014; 13:98. DOI:
10.1186/1476-511X-13-98.
33. Ghasemi N. Therapeutic effects of adipose derived mesenchymal stem cells on remyelination process in inflammatory demyelinating diseases. J Histol Histopathol. 2015; 2:8. DOI:
10.7243/2055-091X-2-8.

34. Abd El Azeem AF, Al-Ahmadyb HM, Abdul Rahmana MA, Abdel Hameed MA, Shaalan OF. Angiogenesis induced by autologous whole bone marrow stem cells seeded on collagen scaffolds in silicone nerve tubes. An experimental study. Tanta Dental J. 2014; 111:227–234. DOI:
10.1016/j.tdj.2014.10.006.

35. Linero I, Chaparro O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One. 2014; 9:e107001. DOI:
10.1371/journal.pone.0107001. PMID:
25198551. PMCID:
4157844.

36. Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, Montorsi F, De Ridder D, Albersen M. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. Eur Urol. 2013; 63:551–560. DOI:
10.1016/j.eururo.2012.09.034.

37. Wang WW, Li ZZ, Wang W, Jiang Y, Cheng J, Lu S, Zhang JY. Enhanced renoprotective effect of HIF-1α modified human adipose-derived stem cells on cisplatin-induced acute kidney injury in vivo. Sci Rep. 2015; 5:10851. DOI:
10.1038/srep10851.

38. Albersen M, Berkers J, Dekoninck P, Deprest J, Lue TF, Hedlund P, Lin CS, Bivalacqua TJ, Van Poppel H, De Ridder D, Van der Aa F. Expression of a distinct set of chemokine receptors in adipose tissue-derived stem cells is responsible for in vitro migration toward chemokines appearing in the major pelvic ganglion following cavernous nerve injury. Sex Med. 2013; 1:3–15. DOI:
10.1002/sm2.1. PMID:
25356281. PMCID:
4184711.

39. Ding Z, Huang H. Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: superiority of meniscus as a cell source for meniscus repair. BMC Musculoskelet Disord. 2015; 16:65. DOI:
10.1186/s12891-015-0511-8. PMID:
25887689. PMCID:
4373281.

40. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011; 109:923–940. DOI:
10.1161/CIRCRESAHA.111.243147. PMID:
21960725. PMCID:
3604746.




 PDF
PDF Citation
Citation Print
Print


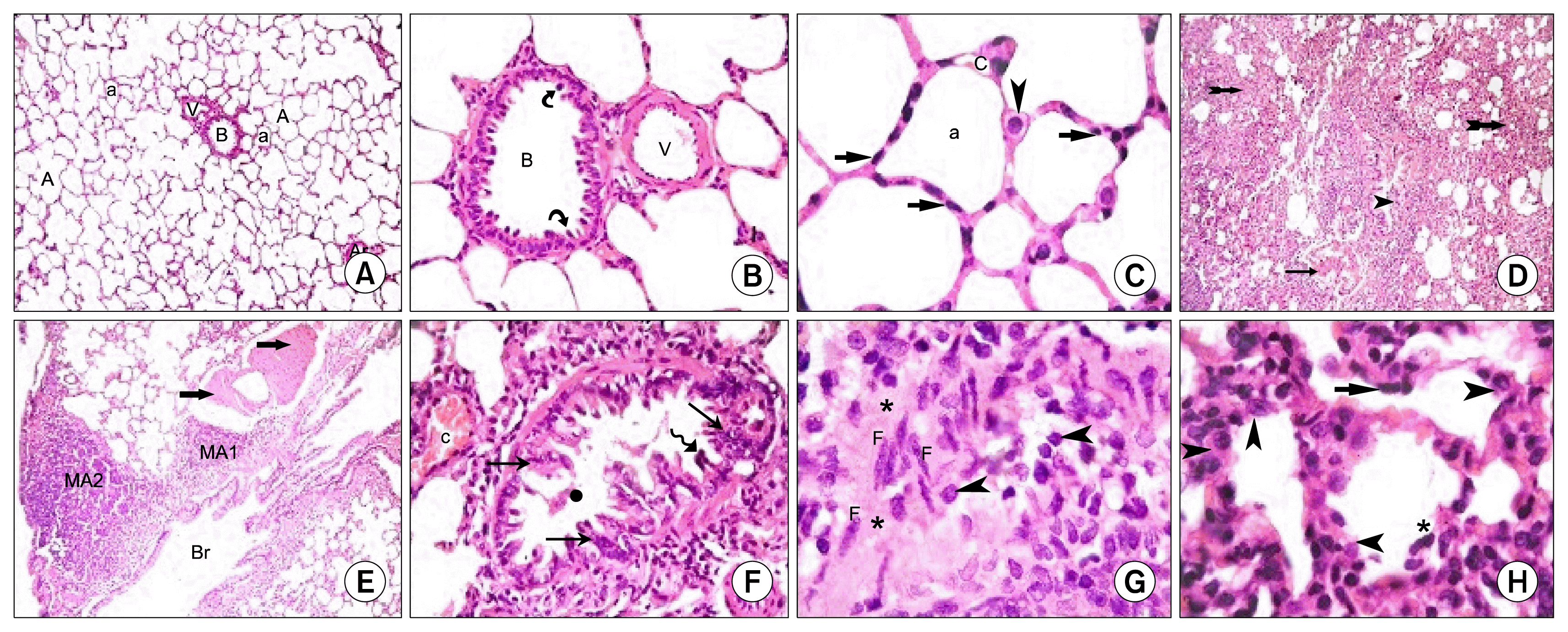
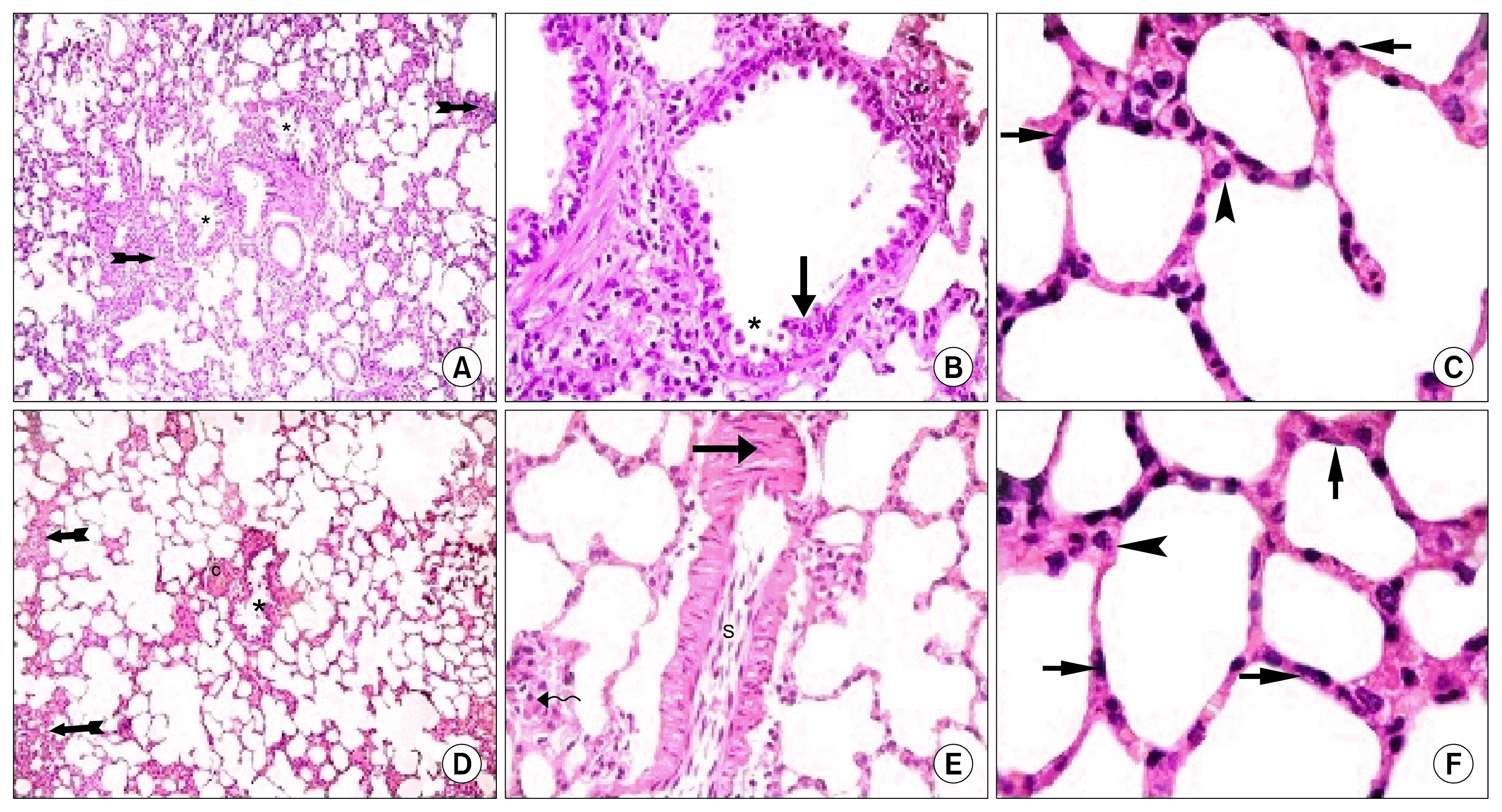
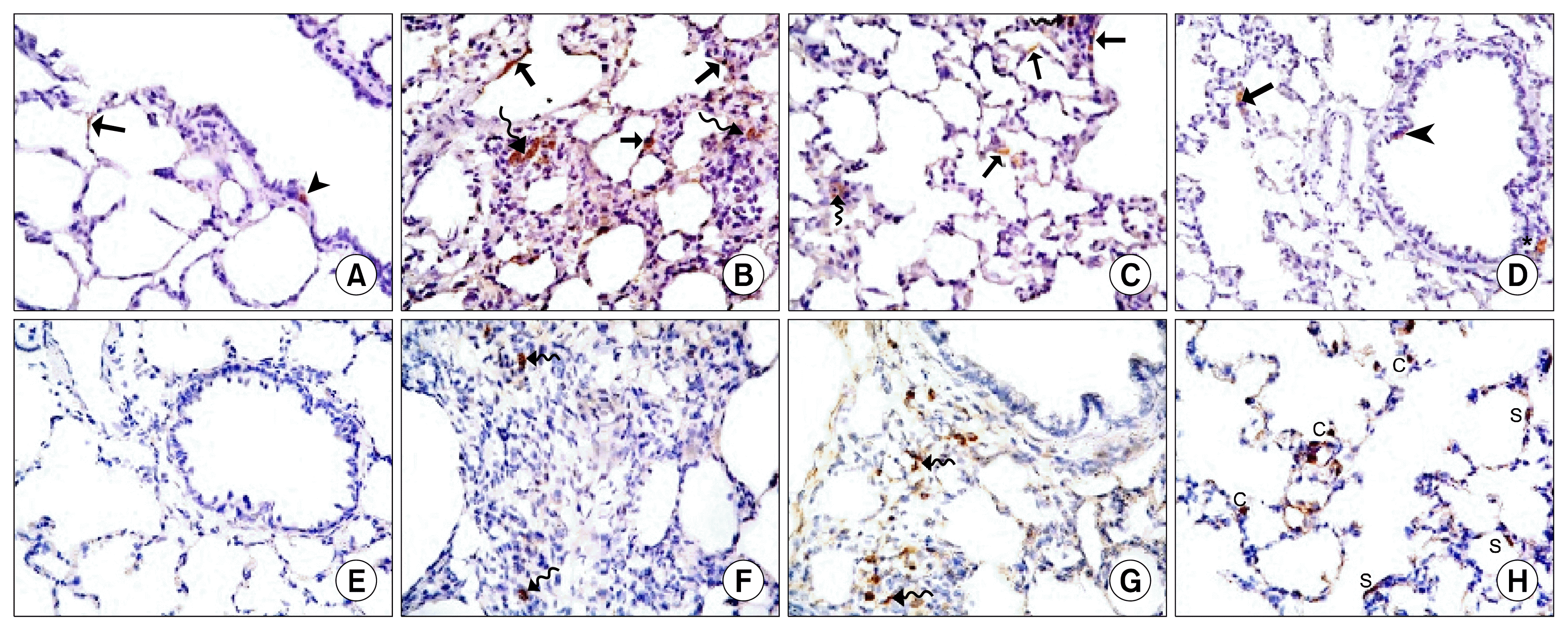
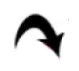 ) (×400) (C): alveoli (a) lined mainly by type I pneumocytes (
) (×400) (C): alveoli (a) lined mainly by type I pneumocytes (
 ). Note few type II pneumocytes (
). Note few type II pneumocytes (
 ) and pulmonary capillaries (c) (×1000). Lung sections in Gp III (H&E) showing (D): obvious thickening of multiple IAS (
) and pulmonary capillaries (c) (×1000). Lung sections in Gp III (H&E) showing (D): obvious thickening of multiple IAS (
 ), a distorted bronchiole (
), a distorted bronchiole (
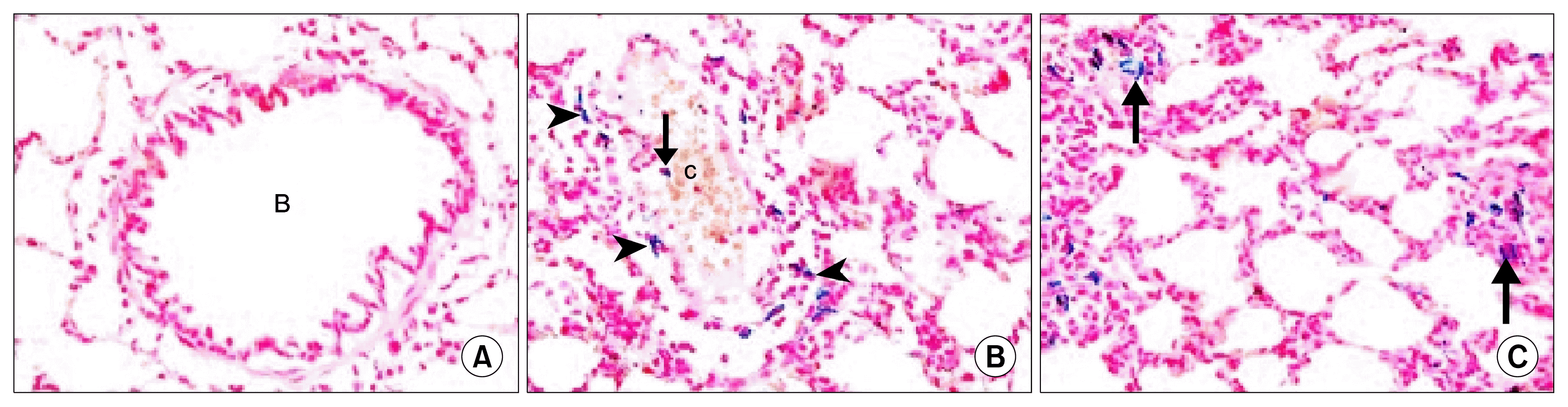
 ) (×100), (E): two peribronchial mononuclear aggregates, (MA1) and (MA2). Note obvious thickening of the wall of a vessel (
) (×100), (E): two peribronchial mononuclear aggregates, (MA1) and (MA2). Note obvious thickening of the wall of a vessel (
 ) in the lumen of a bronchiole, a congested vessel (c). Note the lining cells with dark nuclei (
) in the lumen of a bronchiole, a congested vessel (c). Note the lining cells with dark nuclei (
 ) (×400). (G): thickened IAS harboring multiple fibroblasts (F) surrounded by collagen deposition (*). Note multiple infiltrating cells (
) (×400). (G): thickened IAS harboring multiple fibroblasts (F) surrounded by collagen deposition (*). Note multiple infiltrating cells (
 XML Download
XML Download