Introduction
Materials and Methods
Neurosphere assay
Pan neuronal differentiation
Dopaminergic differentiation
Immunofluorescence analysis
RT-PCR analysis
Nurr1: AGTACCTTTATGGACAACTACAGCA (F), CGTAGTGGCCACGTAGTTCTGGT (R)
PTX3: CTCTCTGAAGAAGAAGCAGCA (F), CCGA GGGCACCATGGAGGCAAC (R)
TH: TCCTGCACTCCCTGTCAGAG (F), CCAAGA GCAGCCCATCAAAGG (R)
DAT: CAGAGAGGTGGAGCTCATC (F), GGCAGA TCTTCCAGACACC (R)
Calbinidin: GCAGTCATCTCTGATCACAGC (F), G AGGTCTGTGTACTCTGCTAG (R)
L-AADC: CCTACTGGCTGCTCGGACTAA (F), GC GTACCAGGGACTCAAACTC (R)
β-actin: GTGGGGCGCCCCAGGCACCA (F), CTC CTTAATGTCACGCACGATTTC (R)
Electrophysiological analysis
Reverse phase HPLC
Generation of hemi-Parkinsonian animals and rotation tests
Transplantation
Statistical Analysis
Results
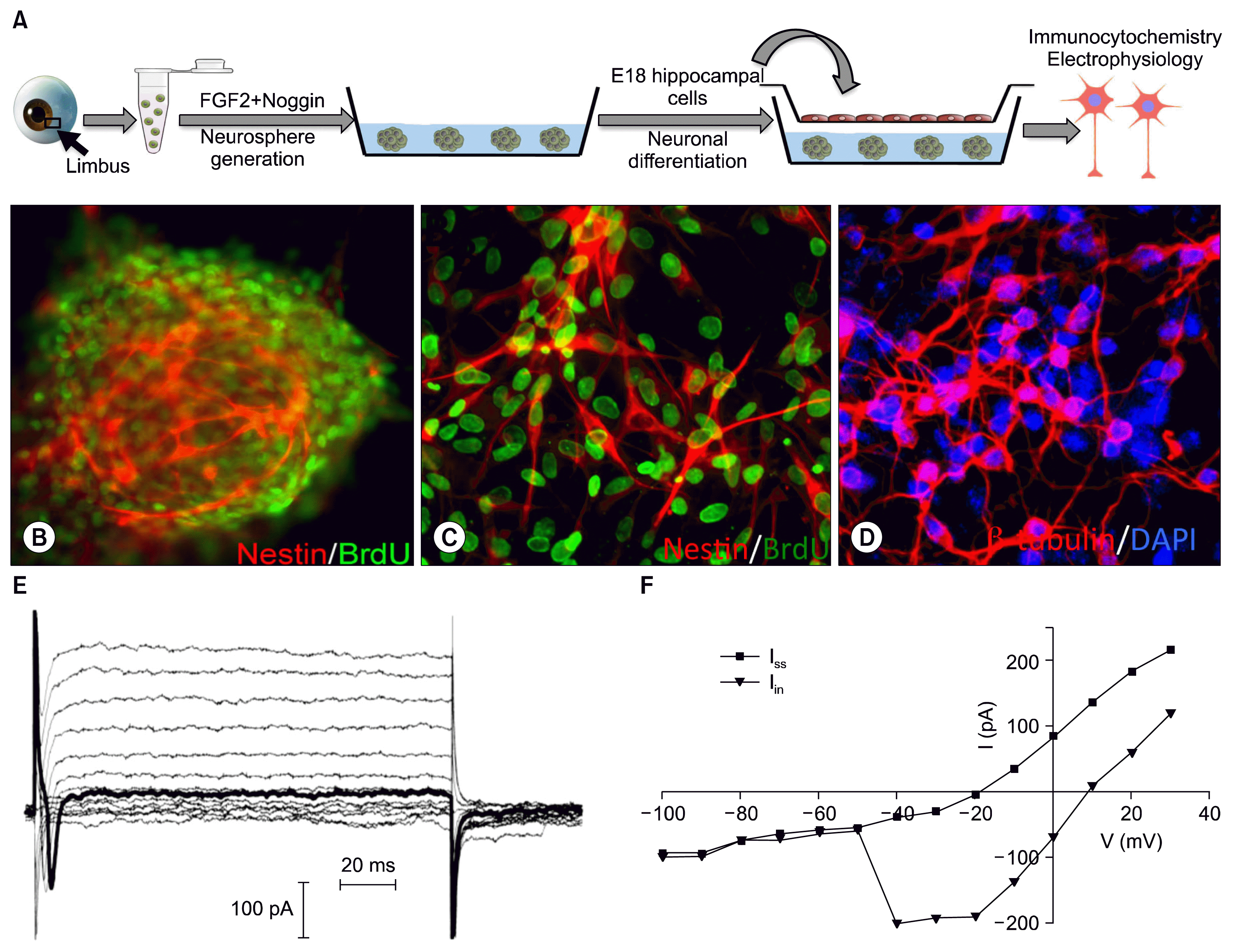 | Fig. 1Generation and differentiation of limbus-derived neural progenitors. A schematic representation of the differentiation protocol is provided in A. Cell dissociates from limbus when cultured in the presence of FGF2 and Noggin generated neurospheres with BrdU+ and Nestin+ cells (B; C, dissociated neurospheres). BrdU-tagged neurospheres, when co-cultured with E18 hippocampal cells spread out and cells acquired neuronal morphology, co-expressing neuronal markers β-tubulin/DAPI (D). Whole cell voltage clamped recordings of differentiated cells with neuronal morphology revealed fast inward currents and sustained outward currents (E). The current-voltage (I~V) curve (F) exhibited a typical I-V relationship of fast inward currents due to the voltage gated Na+ channels and sustained outward currents due to the outwardly rectifying K+ channels. |
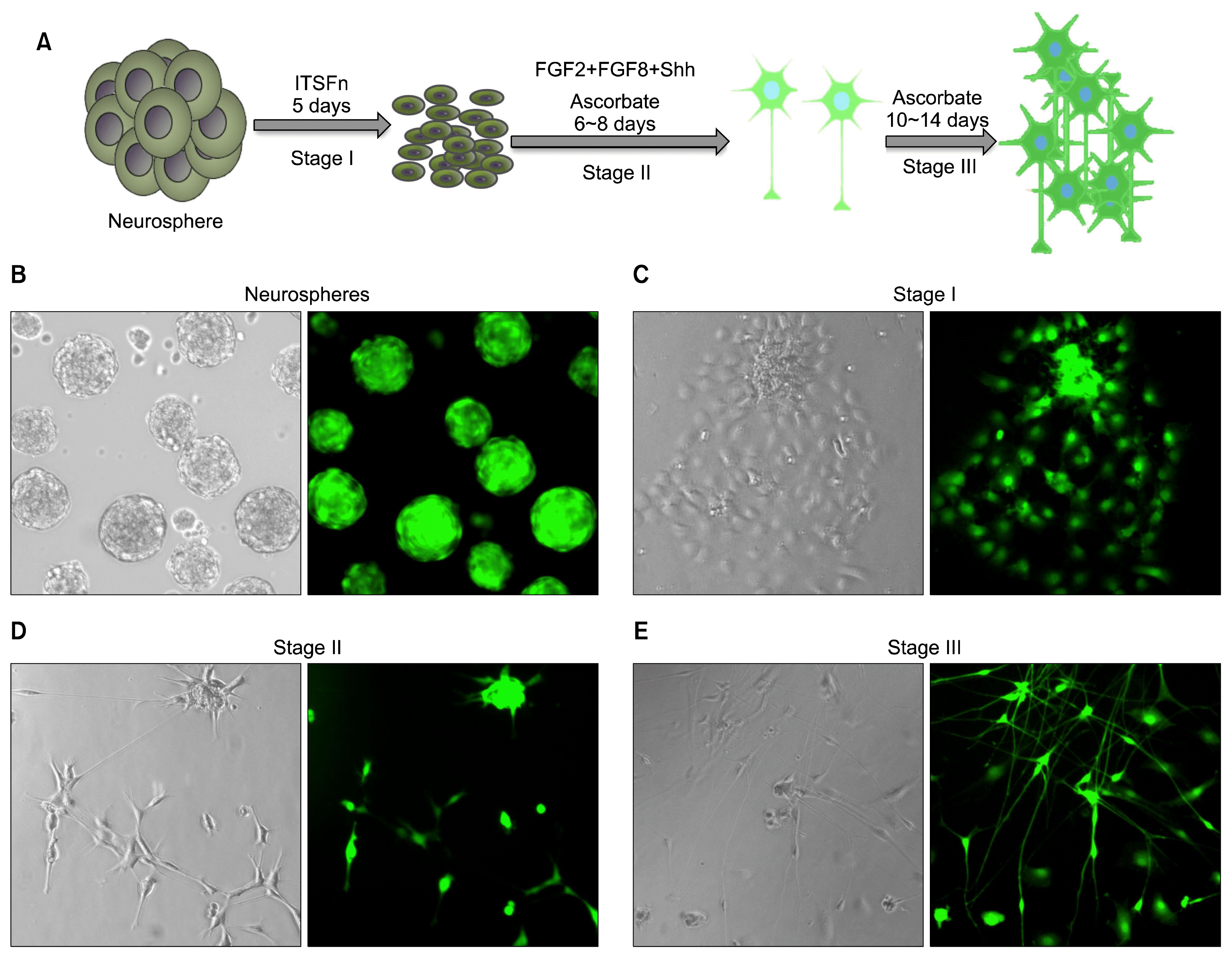 | Fig. 2Differentiation of limbus-derived neural progenitors along the dopaminergic cell lineage. Limbus-derived neurospheres were subjected to three stages directed dopaminergic neuron differentiation protocol, using Shh and FGF8 (A). Cell dissociates from GFP-rat limbus when cultured in the presence of FGF2 and Noggin generated neurospheres with GFP+ neural progenitors. Neurospheres spread under the influence of ITSFn (Stage1) and cells therein acquired neuronal morphology under the influence of Shh and FGF8 (Stage II) and increased in number with ascorbate (Stage III). |
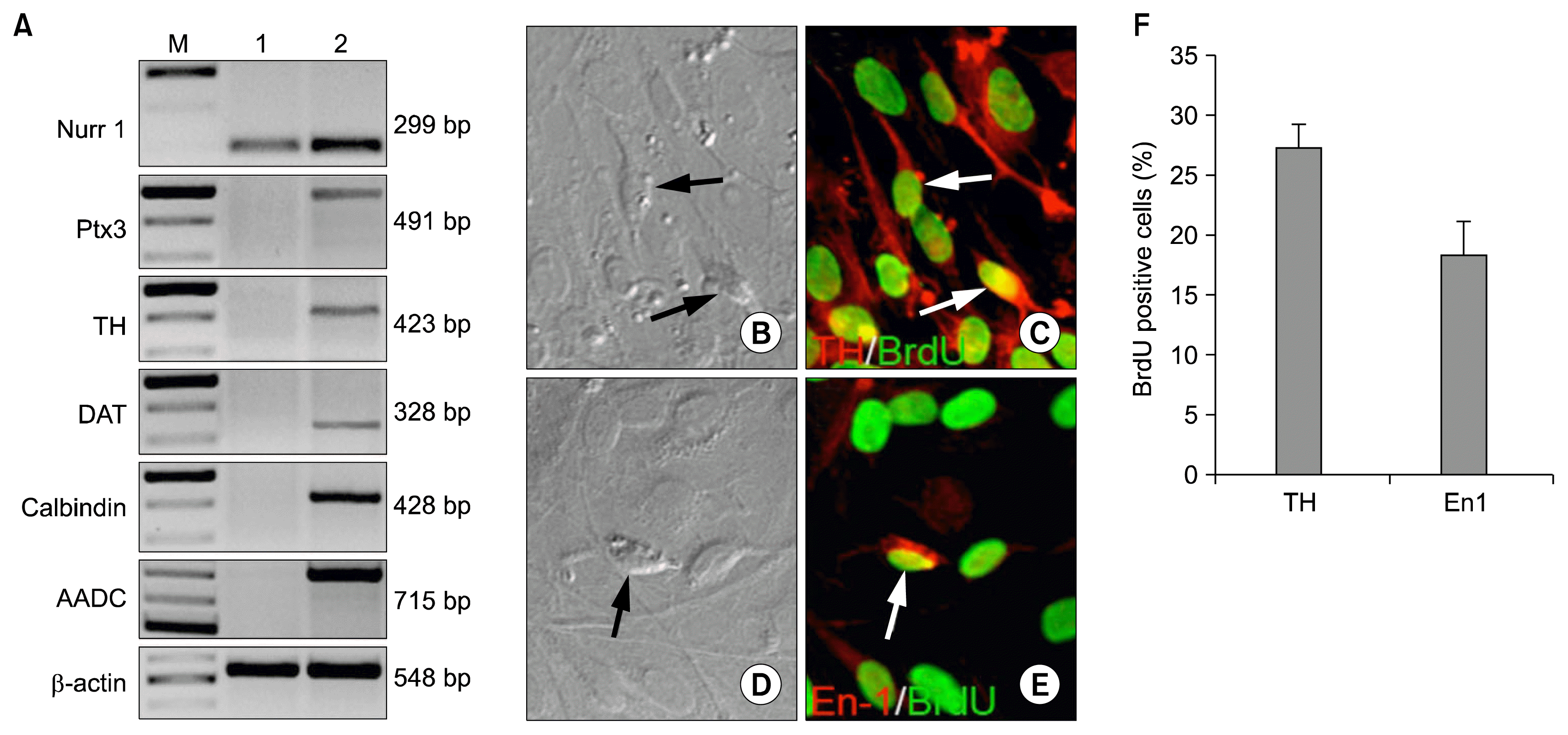 | Fig. 3Molecular and biochemical properties of limbus-derived dopaminergic neurons. RT-PCR analysis of cells in neurospheres (lane 1) and cells at the end of Stage III of differentiation (lane 2) demonstrated the induction of transcripts corresponding to dopaminergic neuron regulator (Nurr1 and Ptx3) and phenotypic and functional markers (Calbindin, TH, DAT, and AADC, compared to the former (A). Immunocytochemical analysis of BrdU-tagged Stage III cells revealed BrdU+ cells, expressing dopaminergic neuron markers, TH (B, C, F) and En1 (D, E, F). |
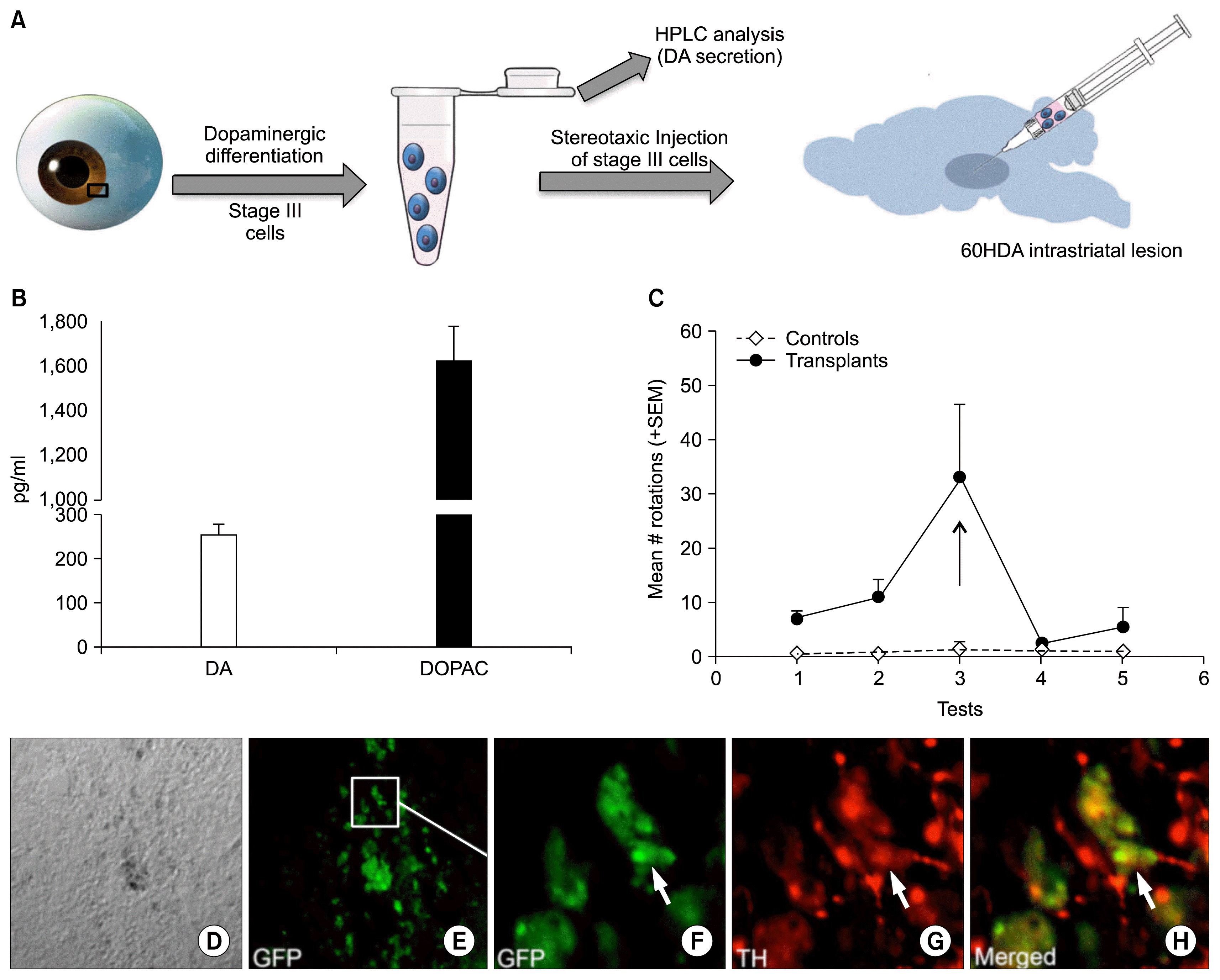 | Fig. 4Functional analysis of limbus-derived dopaminergic neurons. The schematic representation of the functional analysis of non-cell autonomously derived dopaminergic neurons is given in A. the HPLC-EC analysis revealed that limbus-derived Stage III cells release DA and DOPAC in the culture medium in response to KCL (B). Un-induced cells were negative for DA and DOPAC. Transplantation of limbus-derived GFP+ stage III cells in the striatum of 6OHDA-lesioned rats reduced the amphetamine-induced rotations to sham-lesioned controls one-week post-translation (C). Retrospective immunohistochemical analysis of sections through the striatum of transplanted rats revealed GFP+ limbus-derived stage III cells expressing TH in the host’s striatum (D~H). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download