Introduction
Idiopathic pulmonary fibrosis (IPF) is considered one of most common fibrotic lung diseases (1). It is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause limited to the lungs and associated with histopathologic and/or radiologic pattern of usual interstitial pneumonia (UIP) (2). Bleomycin is a chemotherapeutic antibiotic, produced by the bacterium “Streptomyces verticillus” (3). It is an anticancer drug that is used mainly in treatment of Hodgkin, non-Hodgkin lymphomas and testicular carcinoma (4). Bleomycin reproduced typical features of the human interstitial lung fibrosis in the form of intra-alveolar buds, mural incorporation of collagen and obliteration of the alveolar space (5). Myofibroblasts are increased in pulmonary fibrosis and considered principal cells responsible for deposition of collagen and extracellular matrix in lung fibrosis (6). The list of cells from which myofibroblasts can derive has grown impressively during the last years. It includes local fibroblasts, epithelial cells, endothelial cells, smooth muscle cells, pericytes, hepatic perisinusoidal cells, mesenchymal stem cells, and bone marrow-derived cells known as fibrocytes (7). Alpha smooth musle actin is considered a key marker for myofibroblasts (8). Its expression is increased in lung fibrosis and enhance fibro-blast contractility (9). Proliferating cell nuclear antigen (PCNA) is highly conserved between species (10). It serves as a co-factor for DNA polymerase delta in S-phase, as well as during DNA synthesis. The temporal specificity of PCNA expression makes it an ideal marker for cell proliferation (11). Bleomycin was found to increase the expression of PCNA in lung epithelial cells (12) There is an increased evidence supporting the migratory, differentiative and reparative capacity of MSCs in experimental models of lung inflammation and fibrosis (13). They can engraft in injured lung and in some cases are thought to acquire epithelial characteristics (14). This also may explain the several properties of MSCs that include their differentiative, regenerative and migratory capacity, immunomodulation and paracrine activity with the secretion of angiogenic, anti-apoptotic and anti-inflammatory factors (15).
Go to : 
Materials and Methods
Drugs
Bleomycin hydrocloride (Nippon Kayaku, Japan), 15 mg powder per vial was given as a single daily intravenous dose (in the tail vein) of 10 mg/kg mouse/day dissolved in sterile saline for 5 consecutive days (6) and the dose was adjusted according to body weight of animal species according to the paget’s table (16).
Systemically injected MSC: 500,000 cells/150 μl in Dulbecco’s Phosphate Buffered Saline (DPBS, GIBCO, NY, USA) was injected via tail vein immediatly after bleomycin injection (in group III) or seven days after bleomycin injection (in group IV) (17).
Animals: Thirty six adult male albino rats (200∼220 g) were housed in Kasr ElAiny Animal House, Cairo University. divided into four main groups, each group was kept in a separate cage. Anaesthetized with intraperitoneal injection of 75 mg/kg ketamine (intraperitoneal), sacrificed and lungs were dissected out. Procedures were in accordance with institutional guidelines.
Experimental design
Group I: Control (n=6). injected intravenously with saline in tail vein, two rats sacrificed with each experimental group.
Group II: Bleomycin treated rats (n=10). Sacrificed on the 14th day from start of bleomycin injection.
Group III: Combined stem cell and bleomycin treated group (starting together) (n=10) sacrificed on the14th day from the start of bleomycin injection.
Group IV: Bleomycin then stem cell (seven days after bleomycin treatment) treated group (n=10), sacrificed on the14th day from the start of bleomycin injection.
Specimens taken and sectioning: Specimens fixed in 10% buffered formalin solution and processed for paraffin sections of 5∼7 μm thickness, sections mounted on canda balsm coated slides in case of ordinary and special staining and poly-L-lysine coated and charged slides in case of immunostaining. Subjected to following
Immunohistochemistry: For α-SMA antibody and PCNA antibody supplied by NEOMARKER labvision as mouse monoclonal antibody. Anti α-SMA immunohistochemical staining was done according to Bancroft and Cook (20). Paraffin sections were deparaffinized in xylene for 1∼2 minutes and then rehydrated in descending grades of ethanol then brought to distilled water for 5 minutes. Sections were incubated in hydrogen peroxide for 30 minutes then rinsed in PBS (3 times, 2 minutes each). Each section was incubated for 60 minutes with 2 drops (=100 μl) of the primary antibody α-SMA antibody, a Mouse Monoclonal Antibody (Lab Vision Corporation laboratories, CA 94538, USA, catalogue number MS-113-R7). Slides were rinsed well in PBS (3 times, 2 min. each), incubated for 20 minutes with 2 drops of biotinylated secondary antibody for each section then rinsed well with PBS. Each section was incubated with 2 drops (100 μl) enzyme conjugate “Streptavidin-Horseradish per-oxidase” for 10 minutes at room temperature then washed in PBS. Substrate-chromogen (DAB) mixture 2 drops was applied to each section and incubated at room temperature for 5∼10 min. then rinsed well with distilled water. Slides were counterstained with hematoxylin, dehydrated and mounted. α-SMA +ve cells showed brown cytoblasmic deposits. Anti PCNA Immunohistochemical staining was done using PCNA Ab-1 (Clone PC10), a mouse monoclonal antibody (Lab Vision Corporation laboratories, CA 94539, USA, catalogue number MS-106-P). in the same way as α-SMA immunohistochemical staining. PCNA +ve cells showed brown nuclear deposits. All steps performed in a humidity chamber preventing drying of the tissues. Non-specific background elimination step was omitted.
Isolation, culture and labeling of MSCs from rat bone marrow (21): Bone marrow cells obtained from the long bones of 8 weeks old male albino rat by aspiration. Bones flushed with Dulbecco’s Modified Eagle’s medium (DMEM) (Sigma, USA, D5796) supplemented with 10% fetal bovine serum (FBS) (Sigma, USA, F6178). Bone marrow slowly layered over Ficoll- Hypaque (Sigma, USA, F8016) in a ratio of 2:1 in sterile conical tubes and was centrifuged (at 1200 rpm for 30 minutes at room temperature). The opaque layer containing mononuclear cells was aspirated and resuspended in complete culture medium supplemented with 1% penicillin-streptomycin (Sigma, USA, P4333). Cells incubated at 37°C in 5% humidified CO2 for 14 days. Media changed every 3∼4 days. When large colonies developed (80∼90% confluence), cultures washed twice with phosphate buffer saline (PBS) (P5493, Sigma, USA) and cells trypsinized with 0.25% trypsin (Sigma, USA, T1426) in 1 ml Ethylene Diamine Tetra Acetate (EDTA) (Sigma, USA, E6758) for 5 minutes at 37°C. After centrifugation (at 2400 rpm for 20 minutes at room temperature), cell pellets were resuspended with serum-supplemented medium and incubated in 25 cm2 culture flasks (Sigma, USA, C6356). The resulting cultures referred to as first-passage cultures. MSCs in culture were characterized by their plastic adhesiveness and fusiform shape (22).
Morphometric Study
Data obtained using “Leica Qwin 500 C” image analyzer computer system Ltd. (Cambridge, England). The following parameters were measured: area percent of collagen fibers, area percent of α SMA immunopositive cells, optical density of α SMA immunopositive cells, number of pneumocytes type II cells/HPF, Number of PCNA immunostained pneumocytes type II cells/HPF.
statistical analysis: Mean, standard deviation and analysis of variance (ANOVA) were calculated using EXCEL and SPSS 16 software. statistically significant when p was <0.05 (23).
Go to : 
RESULTS
Characteristics of MSCs in culture: MSCs in culture had fibroblast-like morphology and they adhered to the tissue culture substrate within 24∼48 h. They reached confluence within 7∼14 days.
Histological results
Hematoxylin and Eosin stained lung sections: Control group revealed normal normal lung architecture (Fig. 1a). Group II, bleomycin treated rats revealed lost lung architecture in the form of consolidation of lung tissue, that was accompanied by many fibroblasts, dividing pneumocyte type II cells, mitotic figures, fluid exudates (Fig. 1b). Group III revealed normal lung appearance (Fig. 1c). Group IV revealed consolidated tissue with fibroblast cells and some dividing pneumocyte type II cells that exhibited an acinar formation (Fig. 1d).
 | Fig. 1.(1a) A section in the lung of an albino rat (control group), showing alveoli (A) and alveolar epithelium; pneumocyte type I (arrow) and pneumocyte type II with rounded nuclei (arrowhead) (H&E, ×1,000). (1b) A section in a consolidated part of the lung of an albino rat (group II), showing many pneumocyte type II cells (arrows), many fibroblast cells (F), exudates (E) and lymphocytic infiltration (arrow heads). Note the dividing pneumocyte type II cell (D PII) and the mitotic figure (arrow with bifid end) (H&E, ×1,000). (1c) A section in the lung of an albino rat (group III), showing mild thickening of the interalveolar septa with aggregated cells; most probably pneumocyte type II cells (curved arrows) (H&E, ×1,000). (1d) Photomicrograph of a section in a consolidated part of the lung of an albino rat (group IV), showing fibroblast cells (F) and some encircled pneumocyte type II cells with acinar formation (ac) (H&E, ×1,000). |
Masson trichrome stained sections: Sections of control group revealed normal collagen fibers distribution (Fig. 2a). Group II showed extensive collagen fibers deposition (Fig. 2b), group III showed minimal collagen fibers in the lung interstitium and around bronchioles (Fig. 2c). While group IV moderate collagen fibers deposition (Fig. 2d).
 | Fig. 2.(2a) A section in the lung of an albino rat (control group), showing fine collagen fibers within the lung interstitium (arrows) and within the adventia of bronchioles (arrow head) (MT, ×200). (2b) A section in the lung of an albino rat (group II), showing extensive collagen fibers deposition in the lung interstitium and around bronchioles (MT, ×200). (2c) A section in the lung of an albino rat (group III), showing minimal collagen fibers in the lung interstitium (arrow) and around bronchioles (arrow head) (MT, ×200). (2d) A section in the lung of an albino rat (group IV), showing moderate collagen fibers deposition in the lung interstitium (arrows) and around part of a bronchiole (arrow head) (MT, ×200). |
α-smooth muscle actin stained lung section: Sections of control group revealed absence of α-smooth muscle actin immunoreactivity within the lining cells of the alveoli (Fig. 3a). Group II revealed positive α-smooth muscle actin immunoreactivity detected within the cytoplasm of fibroblast cells (Fig. 3b). Group III revealed faint α-smooth muscle actin immunoreactivity within the cytoplasm of pneumocyte type II cells (Fig. 3c). Group IV revealed Positive α-smooth muscle actin immunoreactivity within the cytoplasm of fibroblast cells (Fig. 3d).
 | Fig. 3.(3a) A section in the lung of an albino rat (Control group), showing absence of α-smooth muscle actin immunoreactivity within the lining cells of the alveoli (α-SMA ×1,000). (3b) Another field in the lung of an albino rat (group II), showing positive α-smooth muscle actin immunoreactivity within the cytoplasm of fibroblast cells (F) (α-SMA ×1,000). (3c) A section in the lung of an albino rat (group III), showing very faint α-smooth muscle actin immunoreactivity within the cytoplasm of pneumocyte type II cells (arrows with bifid end) (α-SMA ×1,000). (3d) A section in the lung of an albino rat (group IV), showing positive α-smooth muscle actin immunoreactivity within the cytoplasm of fibroblast cells (curved arrow) (α-SMA ×1,000). |
Proliferative cell nuclear antigen (PCNA) stained lung sections: Sections of control revealed few positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (Fig. 4a). Group II revealed numerous positive PCNA immunoreactivity within nuclei of pneumocyte type II cells and fibroblast cells (Fig. 4b). Group III revealed few positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (Fig. 4c). Group IV revealed many positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (Fig. 4d).
 | Fig. 4.(4a) A section in the lung of an albino rat (Control group), showing few positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (arrow heads) (PCNA ×1,000). (4b) A section of the lung of an albino rat (group II), showing positive PCNA immunoreactivity within the nuclei of fibroblast cells (arrows) and pneumocyte type II cells (arrow heads). Note the dividing pneumocyte type II cells within the lung interstitium (double arrow heads) (PCNA ×1,000). (4c) A section in the lung of an albino rat (group III), showing few positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (arrow head) (PCNA ×1,000). (4d): A section in the lung of an albino rat (group IV), showing many positive PCNA immunoreactivity within nuclei of pneumocyte type II cells (arrow heads) (PCNA ×1,000). |
Detection of PKH26 labeled mesenchymal stem cells by fluorescent microscope in unstained lung sections: Immunofluroscent microscopic examination of lung un-stained section revealed the appearance of red fluorescent PKH26 labeled MSCs within the lung interstitum in group III (Fig. 5a) and heavily distribution of of red fluorescent PKH26 labeled MSCs within the consolidated lung interstitum in group IV (Fig. 5b).
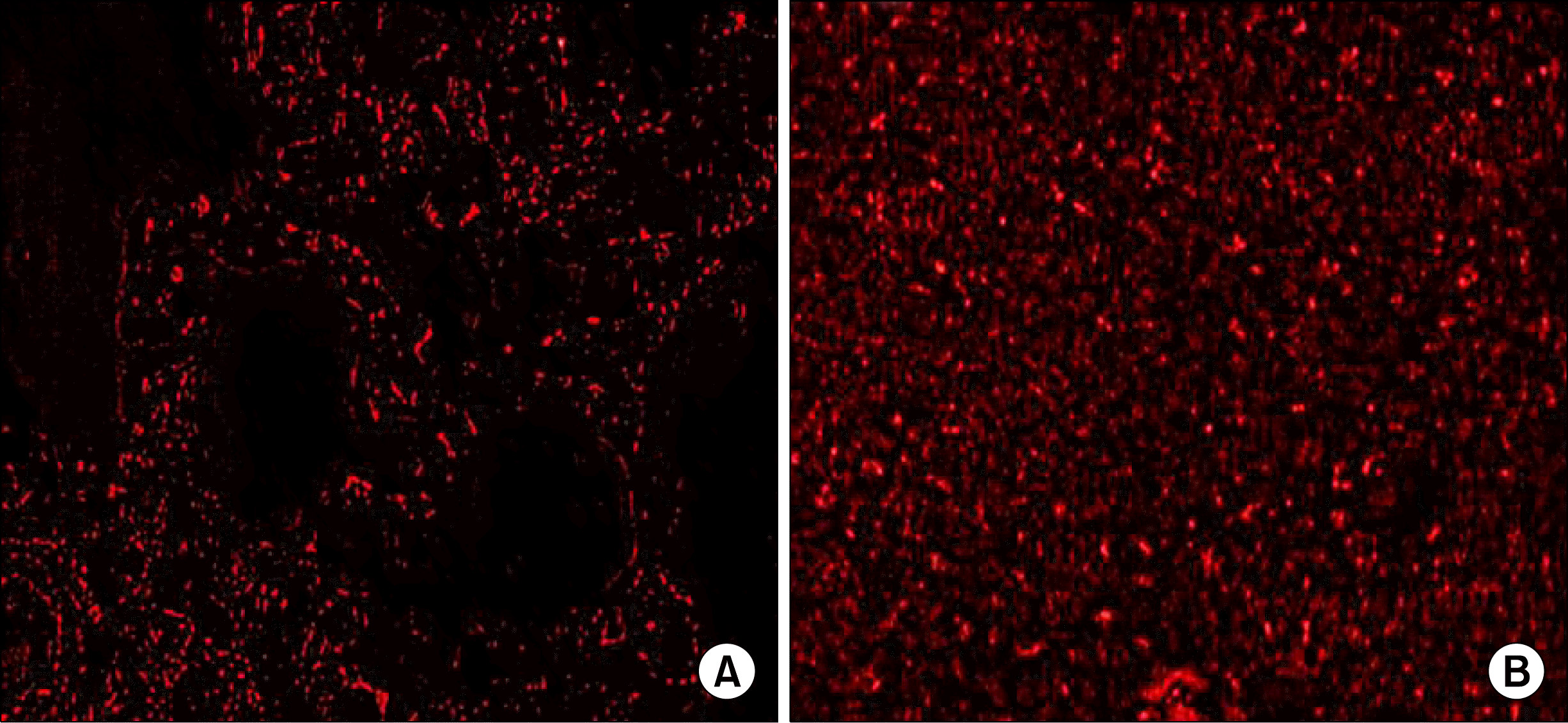 | Fig. 5.(5a) Fluorescent micrograph of unstained section in the lung of an albino rat (group III), showing red fluorescence of PKH26 labeled MSCs within the lung interstitum (PKH26 labeled MSCs immunofluorescence ×400). (5b) Fluorescent micrograph of an another unstained section in the lung of (group IV), showing red fluorescence of PKH26 labeled MSCs, heavily distributed within the consolidated lung interstitum (PKH26 labeled MSCs immunofluorescence ×400). |
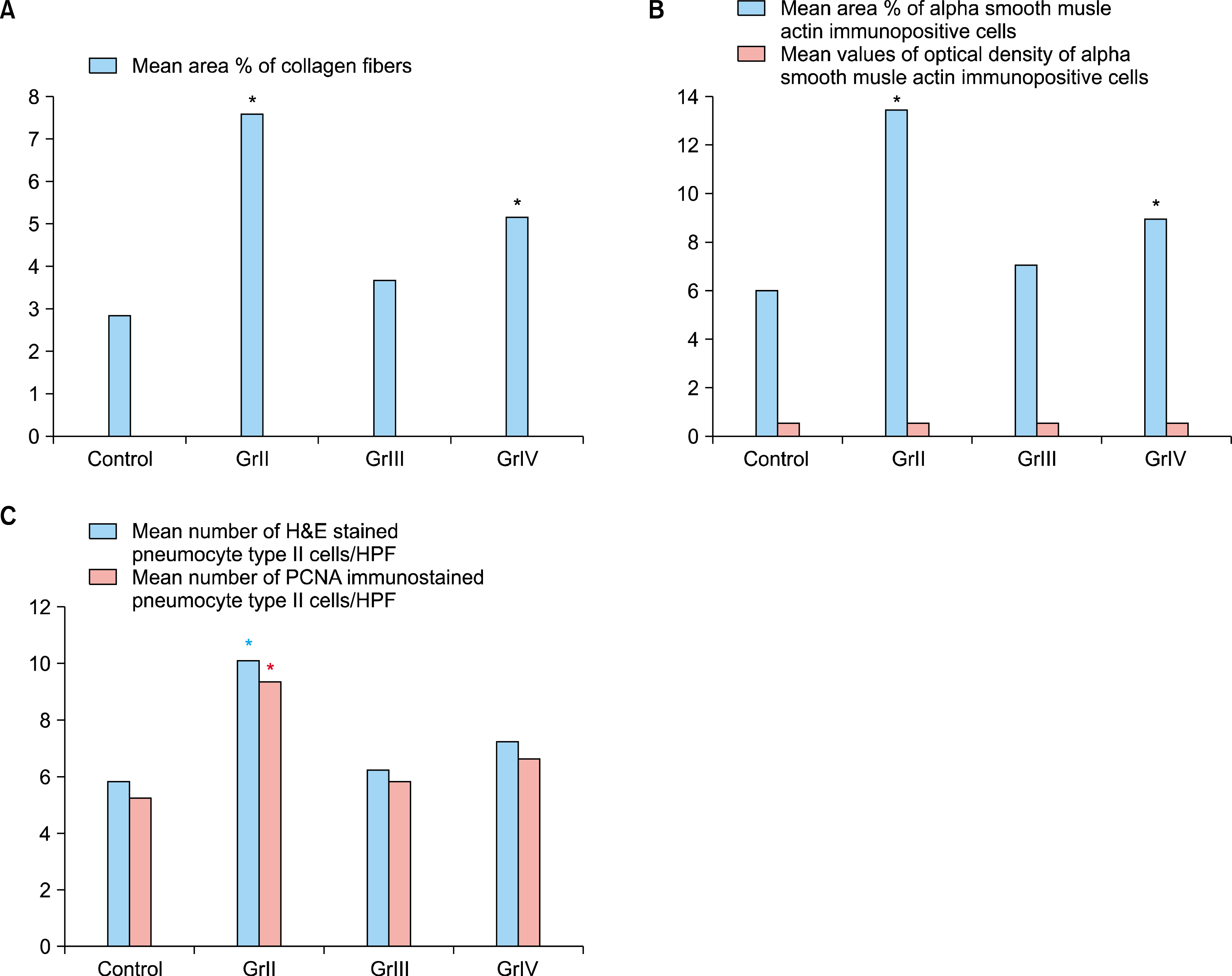
Morphometric results: (Fig. 3 & Table)
I. Mean area percent of collagen fibers: The mean area percent of collagen fibers in Masson’s trichrome stained sections in the control group was (2.9±1.0). It showed an increase in groups II &IV to reach (7.6±1.29) & (5.2± 0.84) that was significantly increased when compared with group I & III. In group III it was (3.7±1.0) that showed non significant difference when compared with group I.
II. Mean values of area percent & optical density of α-SMA immunopositive cells: There was an increase in the mean area percent of α– SMA immunopositive cells stained sections in groups II & IV to reach (13.6±1.5) & (9.1±1.1) that was significantly increased when compared with group I & III. In group III it was (7.2±1.5) that showed non significant difference when compared with group I (6.12±1.7). On the other hand, the mean optical density of α –SMA immunopositive cells in the control group was (0.59±0.45) while in group II, III & IV was (0.635±0.36), (0.58±0.30) & (0.621±0.84) respectively with non significant difference when compared with the control group.
III. Mean number of pneumocyte type II cells in H&E as well as PCNA immunostained in the studied groups: In H&E stained sections the mean number of pneumocytes type II cells in the control group was (5.91±2.23). There was an increase in group II to reach (10.2±2.57) which showed a significant increase when compared with all studied groups. While the mean number of pneumocytes type II cells in group III & IV was (6.3±2.8) & (7.3± 4.0) respectively which was statistically non significant when compared with the control group. On the other hand, regarding PCNA immunostained sections, the mean number of pneumocytes type II cells in the control group was (5.3±2.00). There was an increase in group II to reach (9.4±1.17) which showed a significant increase when compared with all studied groups. While the mean number of pneumocytes type II cells in group III & IV was (5.9±2.18) & (6.7±1.25) respectively which was statistically non significant when compared with the control group.
Table 1.
Mean values of area percentage of collagen fibers, area percent & optical density of α– SMA immunopositive cells and mean number of H&E & PCNA stained pneumocyte type II cells in the studied groups
Go to : 
Discussion
The aim of this study is to demonstrate the bleomycin induced histological changes in the lung and the possible protective and/or therapeutic effect of stem cell therapy against these changes.
The present work showed that (I.V) administration of bleomycin at a dose of 7 mg/kg/day for five consecutive days followed by sacrificing rats at day 14 of experiment (in group II) produced an inflammatory reaction in the form of thickening of septa accompanied by an infiltration of the lung tissue with inflammatory cells such as macrophages and lymphocytes, rather than many observed fibroblasts. It could be assumed that the inflammatory cells especially macrophages and lymphocytes may play an important role in activation of such fibroblasts and subsequent fibrosis. These finding were concomitant with (24) who reported that bleomycin can promote acute cellular inflammation in lung tissue as demonstrated by a strong influx of inflammatory cells such as macrophages and activation of fibroblasts. Group II showed a significant increase in the number of pneumocyte type II cells (AECII) in both H&E and PCNA immunostained sections when compared to both control group and group III. Acinar formation were detected in certain fields, this could be explained by an attempt of these cells to repair. This is in accordance with (25) who defined AECII as local progenitor cells that can accomplish epithelial repair and regeneration in the different regions along the respiratory tract. It could be suggested that these cells might be a source of the myofibroblasts. This suggestion could be supported by the work of (26) and (24) who reported that epithelial-mesenchymal transition (EMT) is the process by which an epithelial cell becomes a more motile mesenchymal cell.
Confirming results of section stained with H&E&Masson,s trichrome stains, group II revealed a significant increase in the mean area percent of α-SMA immunoreactive myofibroblast as compared to the control group & group III, which may be explained by the ability of bleomycin to increase the number of the myofibroblasts. In aggrement with the previous finding, (27) found that bleomycin treatment significantly led to upregulation of pro-fibrotic genes such as α-SMA, which is considered as a key marker for myofibroblasts.
Current work showed that early combination of stem cell therapy with bleomycin treatment (in group III) revealed normal lung appearance that may be a direct relieving effect of early stem cell therapy on bleomycin treatment. This was supported by non significant increase in the mean area percentage of both collagen fibers & α-SMA immune positive myofibroblasts as well as the mean number of pneumocyte type II cells in both H&E and PCNA stained section in group III comparing with control. (17) further added that early treatment with MSCs may produce antagonists to tumor necrosis factor or other cytokines that disrupt signal pathways reducing the extent of inflammation within the lung. This was evidenced in our study by large areas of undamaged tissue with normal alveolar architecture. (28) commented that acceleration of resolution of fibrosis is by epithelial restitution. (29) also stated that MSCs restore cytoskeletal reorganization in alveolar epithelial cells. Current work also showed that late combination of stem cell therapy with bleomycin treatment in group IV revealed a significant increase in the mean area % of collagen fibers and α-SMA immunoreactive myofibroblasts when compared with both control group and group III. This may due to non protective effect of late combined stem cell therapy. In agreement with (17) who reported that, delaying MSC administration by 7 days after BLM challenge has not significantly reduced the hydroxyproline and collagen content of the lung therefore it could be noted that delaying injection of MSCs did not inhibit engraftment but eliminated the ability of the cells to alter the course of disease progression. Therefore, MSCs may produce factors that impinge on molecules expressed early but not late during the course of BLM-induced injury. However, (30) reported that both early (1 day) and late (7 day) MSC administration after BLM challenge has significantly reduced the hydroxyproline and collagen content of the lung but they stated that the early treatment was more significant and effective than the late treatment.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download