Introduction
Materials and Methods
Material
Drugs:
- Cyclophosphamide (CY) (Trade name Endoxan), was purchased from Baxter Oncology GmbH, Halle, Germany, in the form of a vial 200 mg. The drug was dissolved in 10ml phosphate buffered saline (PBS) solution and was given by intraperitoneal (I.P.) injection, at a dose of 50 mg/kg/day (6).
- AstragalusMembranaceus (AM) Extract (Traditional Chinese Medicinal Herbs), manufactured by NOW FOODS 395 S. Glen Ellyn Rd., Bloomingdale, IL 60108. Made in U.S.A. It is supplied in a capsule form. Each capsule contains 500 mg of dried extract of AM (root). Each capsule was dissolved in 5 ml PBS vehicle and was supplied orally, at a dose of 2.5 g/kg body weight by the use of a special blunt tipped syringe (6).
They were divided into the following groups:
- Group I (GI) Control Group included 6 rats that received a vehicle of PBS solution, in the same pattern and route of administration (oral and/or I.P.) as the corresponding experimental groups and subgroups.
- Group II (GII) included 12 rats which were injected I.P. with CY 50 mg/kg/day (0.5 ml for each rat) for 3 days.
gIIa: 6 rats were sacrificed the following day after receiving the last dose of CY.
gIIb: 6 rats continued for one more week to receive 2.5 g/kg/day AM extract orally (5 ml/day).
- Group III (GIII) included 6 rats which received CY I.P. (50 mg/kg/day) together with 2.5 g/kg/day AM orally for 3 days.
- Group IV (GIV) included 12 rats which all received 2.5 g/kg/day AM orally for one week.
Methods
Laboratory Investigations:
- Retro-orbital blood samples were collected by capillary tubes for analysis of Total Leucocytic Count (TLC) and Lymphocytic Count (LC). This was performed in the Clinical Pathology Department, Kasr Al-Ainy Medical Hospital.
- Detection and Counting of haemopoietic stem cells using flowcytometry with CD34 antibody (a commonly used marker for haemopoietic progenitor cells of all lineages) (9). CD34 was measured in the bone marrow samples by flowcytometry in Kasr Al Ainy Hospital, Flowcytometry Unit, Clinical Pathology Department.
Sections were subjected to the following stains:
Morphometric Study
- Mean area % of cellular bone marrow regions occupied by developing haemopoietic cells. The area percent represented the % of the cellular bone marrow regions occupied by developing haemopoietic cells, which were outlined and masked by a binary color to the area of the standard measuring frame. It was measured using an objective lens ×10 i.e. a total magnification ×100.
- Mean area of fat cells in bone marrow sections. From the interactive measurement menu, “measure area” was selected. The cursor of the mouse was used to draw the outline of fat in each field and then the total area of fat for the field was calculated. It was measured at a magnification ×100 in 10 serial fields in each section.
- Mean number of CD20 immunopositive B lymphocytes in the bone marrow.The cursor of the mouse was used to count the number of CD20 +ve cells and the mean value of number of cells for each slide was obtained. It was measured at a magnification ×400, using the interactive measure menu.
Statistical Analysis
Results
Results of Laboratory Investigations
Total Leucocytic Count (TLC) (Table 1 & Fig. 1A):
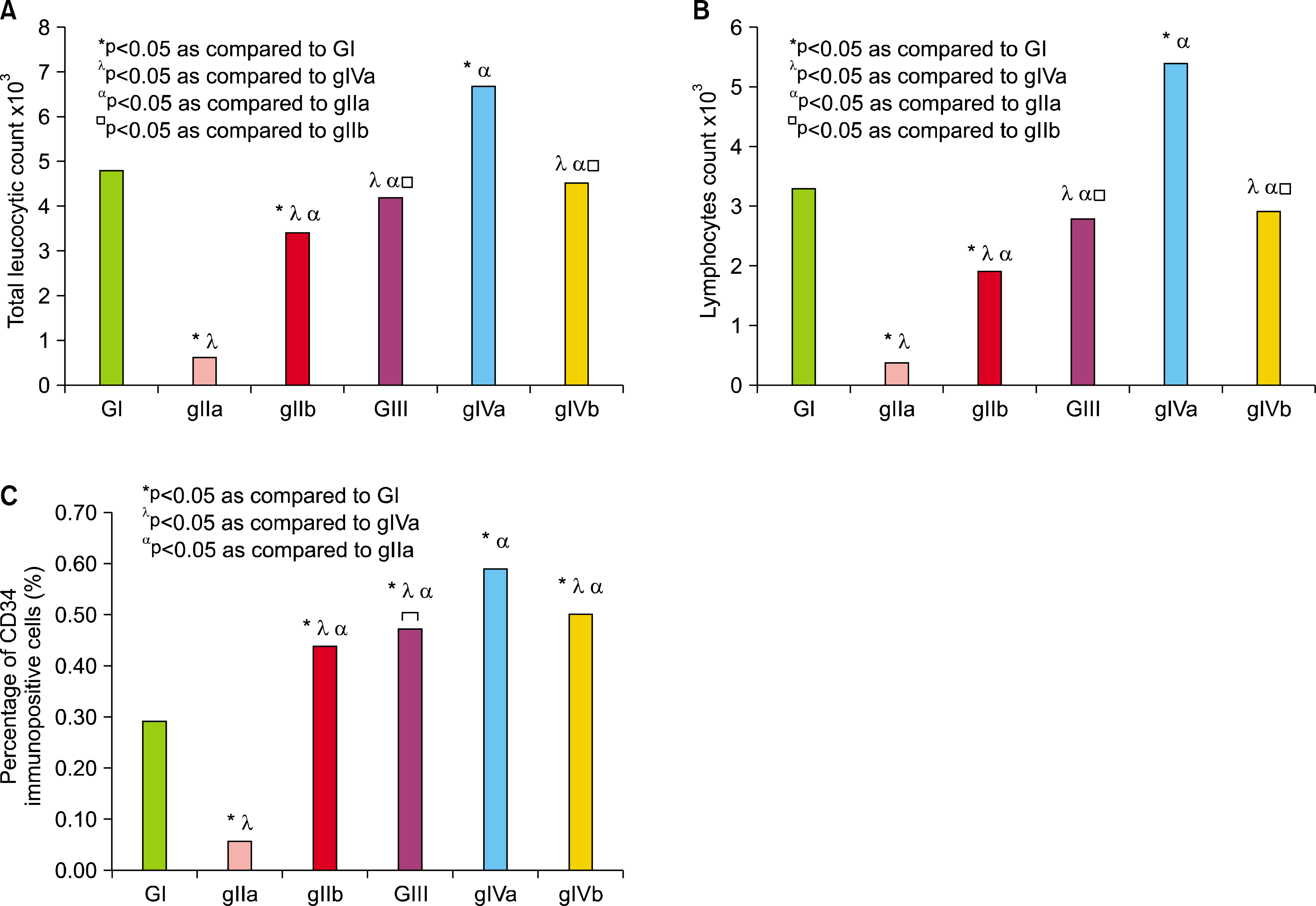 | Fig. 1.(A) Comparing the mean values of TLC in the blood of control and experimental groups. (B) Comparing the mean values of lymphocytic countin the blood of control and experimental groups. (C) Comparing the mean values of the percentage of bone marrow CD34 immunopositive cells by flowcytometry. *p<0.05 as compared to GI, λp<0.05 as compared togIVa, αp<0.05 as compared to gIIa, □p<0.05 as compared to gIIb. |
Histological Results
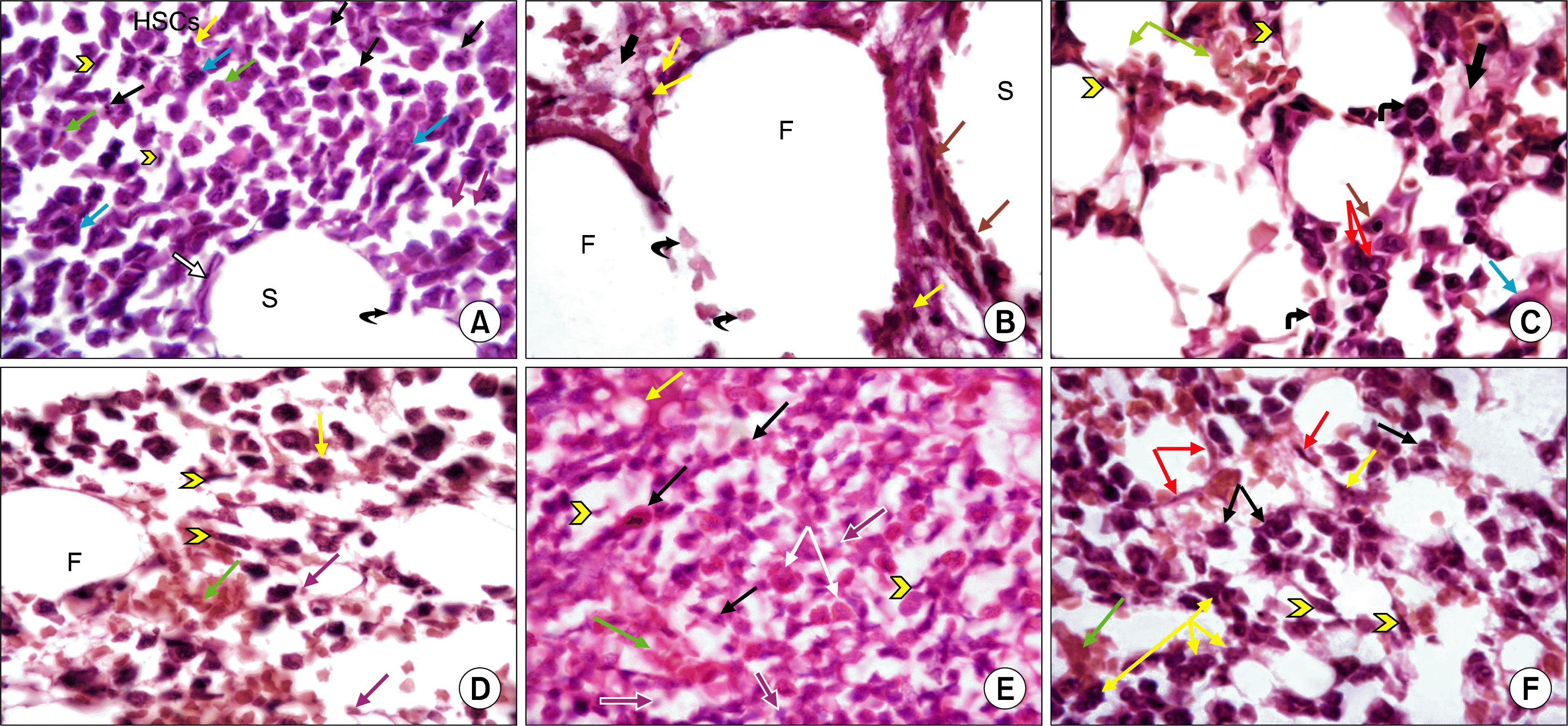 | Fig. 2.(A) A photomicrograph of a transverse section (T.S) in the mid-shaft of femur from control group (GI) showing developing HSCs. Some cells are irregular in outline and show mitotic figures (black arrows), other cells arespindle-shaped (arrow heads). Different stages of myeloid cells are predominant, as well as erythroid cells (green arrows) in addition to megakaryocytes (blue arrows) and macrophages (yellow arrow). Pale nuclei in the background belong to adventitial reticular cells (purple arrows). A blood sinusoid (S) is demonstrated lined by endothelial cells (white arrow). Note interruption of the wall of the sinusoid by a cell passing through it (curved arrow) (H&E, ×1,000). (B) A photomicrograph of a transverse section (T.S) in the mid-shaft of femur fromgIIa showing BM cavities displaying large sized fat cells (F). A dilated blood sinusoid (S) is lined with hypertrophied endothelial cells (brown arrows). Several large branched cells containing engulfed black deposited material, probably macrophages, are seen (yellow arrows). Note the presence of acidophilic granular exudate material in the stromal background (thick arrow) and the presence of several damaged adventitial cells (curved arrows) (H&E, ×1,000). (C) A photomicrograph of T.S in the midshaft of femur from gIIb showing improved bone marrow cellularity, with presence of more developing HSCs. Some of HSCs are spindle-shaped (arrow heads), others have irregular outline and exhibit large nuclei (curved arrows), while others exhibit pyknotic nuclei (brown arrow). Some cells show cytoplasmic vacuolations (red arrows). Note erythroid cells and RBCs (green arrows). Megakaryocyte (blue arrow) is the largest cell seen, characterized by having lobulated nucleus and abundant cytoplasm.Acidophilic exudate material is seen in the intercellular spaces (thick arrow) (H&E, ×1,000). (D) A photomicrograph of T.S in the midshaft of a femur from GIII showing groups of developing HSCs; some of which are spindle shaped (arrow heads) and erythroid cells (green arrow), among oval shaped fat cells. Nuclei of the developing cells are large and darkly stained, while pale nuclei in the background belong to adventitial reticular cells (purple arrows). Note the presence of a multi-nucleated phagocytic cell (yellowarrow). A fat cell (F) is seen bounded by adventitial reticular and haemopoietic cells (H&E, ×1,000). (E) A photomicrograph of T.S in the midshaft of femur from gIVa showing overcrowding of the bone marrow cavity with several developing HSCs exhibiting prominent nuclei. Many cells are spindle-shaped (arrow heads), others exhibit mitotic figures (black arrows). Numerous eosinophils are observed (white arrows). Spaces occupied by fat cells are small but clearly seen between HSCs (yellow arrow). Pale nuclei in the background belong to adventitial reticular cells (purple arrows). A longitudinal section in a small blood vessel is detected engorged with RBCs (green arrow) (H&E, ×1,000). (F) A photomicrograph of T.S in the midshaft of femur from gIVb showing moderate population of bone marrow with developing HSCs, some of which are spindle-shaped (arrowheads), others showmitotic figures (black arrows). Blood sinusoids are seen lined by endothelial cells (red arrows), full with RBCs (green arrow) and WBCs, while macrophages (yellow arrows) are seen bordering blood sinusoids (H&E, ×1,000). |
Immunohistochemical Results
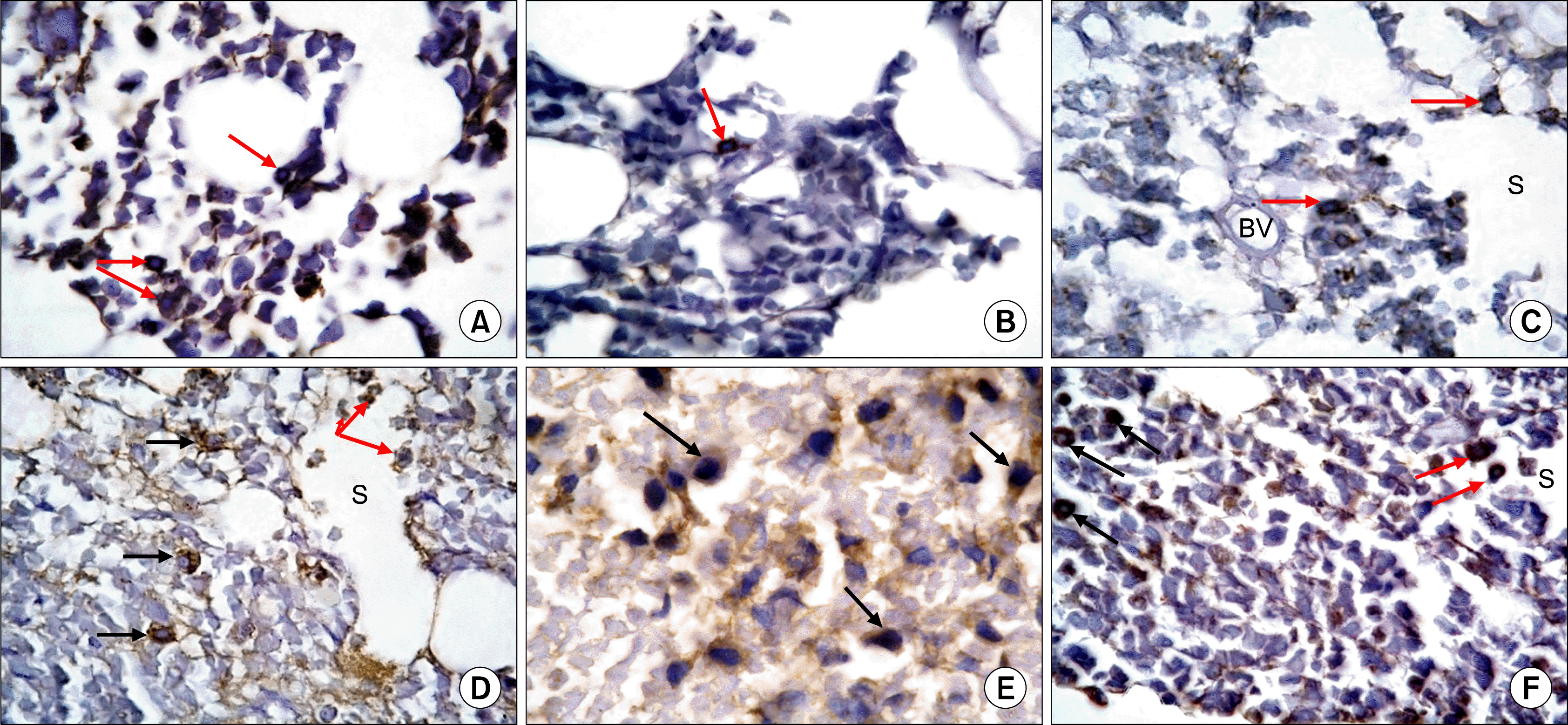 | Fig. 3.(A) A photomicrograph of T.S in the midshaft of femur from the control group (GI) showing brown granular cytoplasmic immunoreactivity of some cells (arrows) (Anti-CD20 Immunostaining ×1,000). (B) A photomicrograph of T.S in the midshaft of femur from gIIa showing a CD20 immunoreactive cell exhibiting brown cytoplasmic granules (arrow) (Anti-CD20 Immunostaining ×1,000). (C) A photo-micrograph of T.S in the midshaft of femur from gIIbshowingCD20immunoreactive cells exhibiting diffuse brown cytoplasmic granules (arrows). Note grouping of cells between a blood vessel (BV) and a blood sinusoid (S). (Anti-CD20 Immunostaining ×1,000). (D) A photo-micrograph of T.S in the midshaft of femur from GIIIshowing diffuse, brown granular cytoplasmic immunoreactivity in many cells (black arrows) closely related to blood sinusoid. Immunoreactive cells are also seen inside the lumen of a sinusoid (S), some are crossing the sinusoidal wall (red arrows) (Anti-CD20 Immunostaining ×1,000). (E) A photomicrograph of T.S in the midshaft of femur from gIVashowing brown granular cytoplasmic immunoreactivity in many cells (arrows). Cells have rounded or oval nuclei (Anti-CD20 Immunostaining ×1,000). (F) A photomicrograph of T.S in the midshaft of femur from gIVb showing diffuse brown granular cytoplasmic immunoreactivity of some cells (black arrows). Some of the cells (redarrows) are seen crossing the blood sinusoid (S) (Anti-CD20 Immunostaining ×1,000). |
Quantitative Morphometric Results
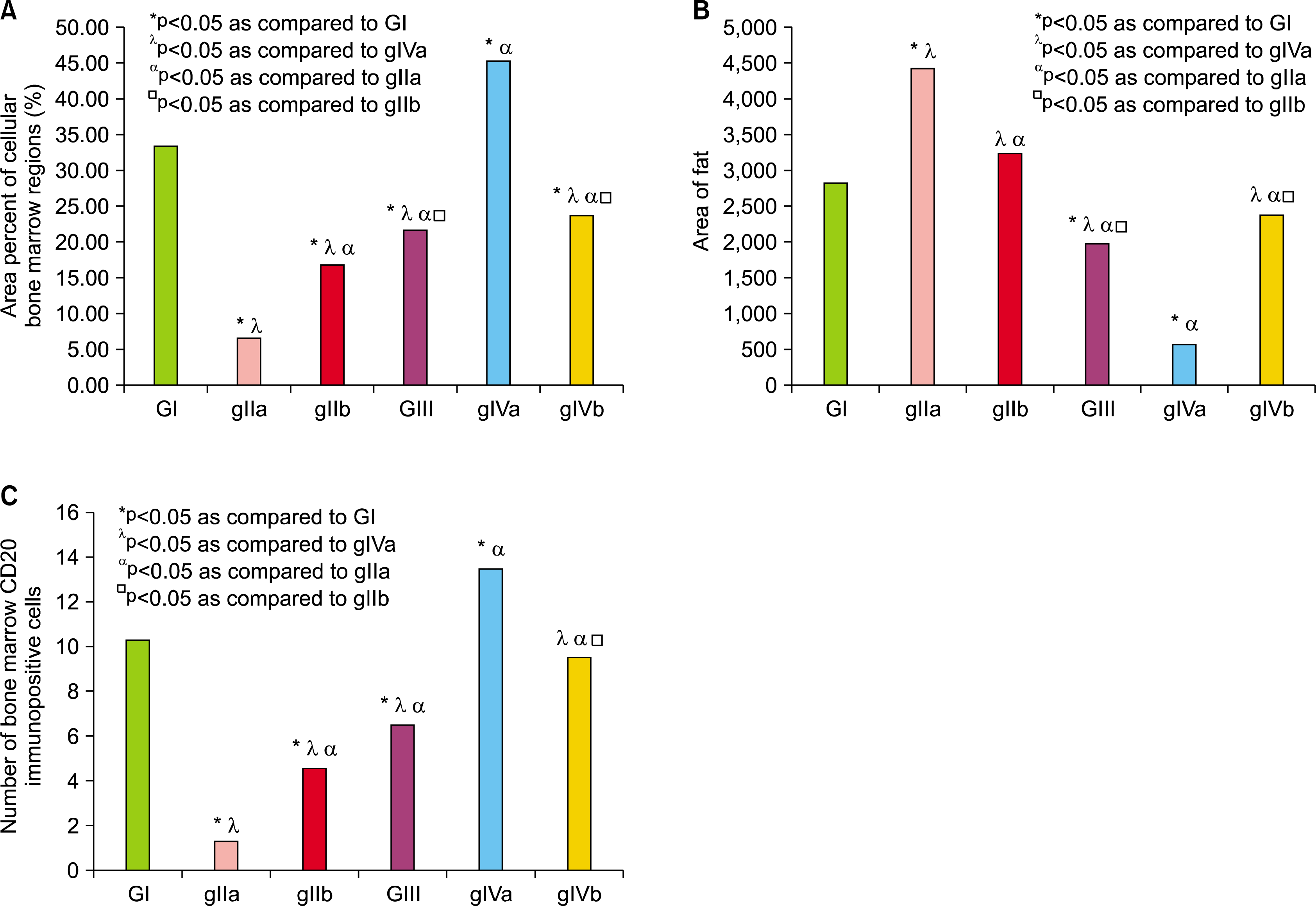 | Fig. 4.(A) Comparing the mean values of mean area percent of cellular bone marrow regions. (B) Comparing the mean values of area of fat cells in the control and experimental groups. (C) Comparing the mean values of bone marrow CD20 immuno-positive cells number in the control and experimental groups. *P <0.05 as compared to GI, λP < 0.05 as compared togIVa, αP <0.05 as compared to gIIa, □P<0.05 as compared to gIIb. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download