Introduction
Materials and Methods
hMSC preparation and culture
Feridex incorporation in Hmsc
Characterization of hMSC
hMSC differentiation assays
Prussian blue staining protocol for iron
Detection of osteogenic, adipogenic and chndogenic differentiation
Immunostaining assay for in vitro neural differentiation
In vitro proliferation of lymphocytes
Results
Feridex-labeled hMSC show typical MSC phenotype
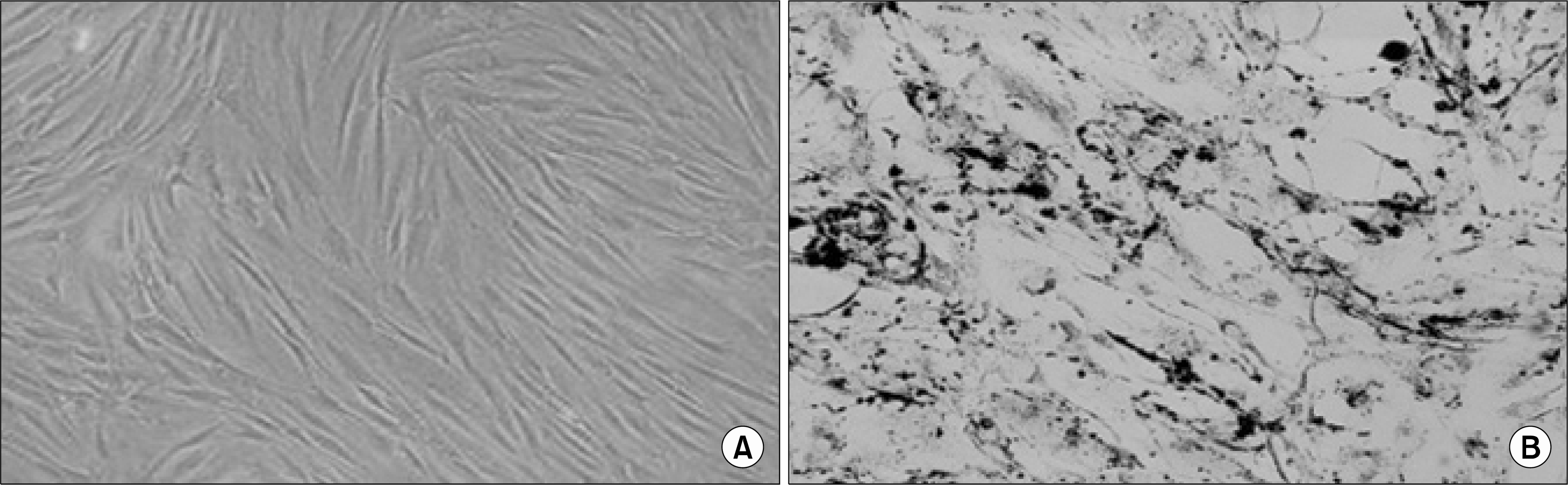 | Fig. 1.Labeling of hMSCs with Feridex. (A) naïve hMSC isolated on day 14 after BM seeding, showing the classical fibroblast-like spindle shaped of the cells. (B) Positive Prussian Blue staining of hMSC cultured with Feridex for 48 hrs. |
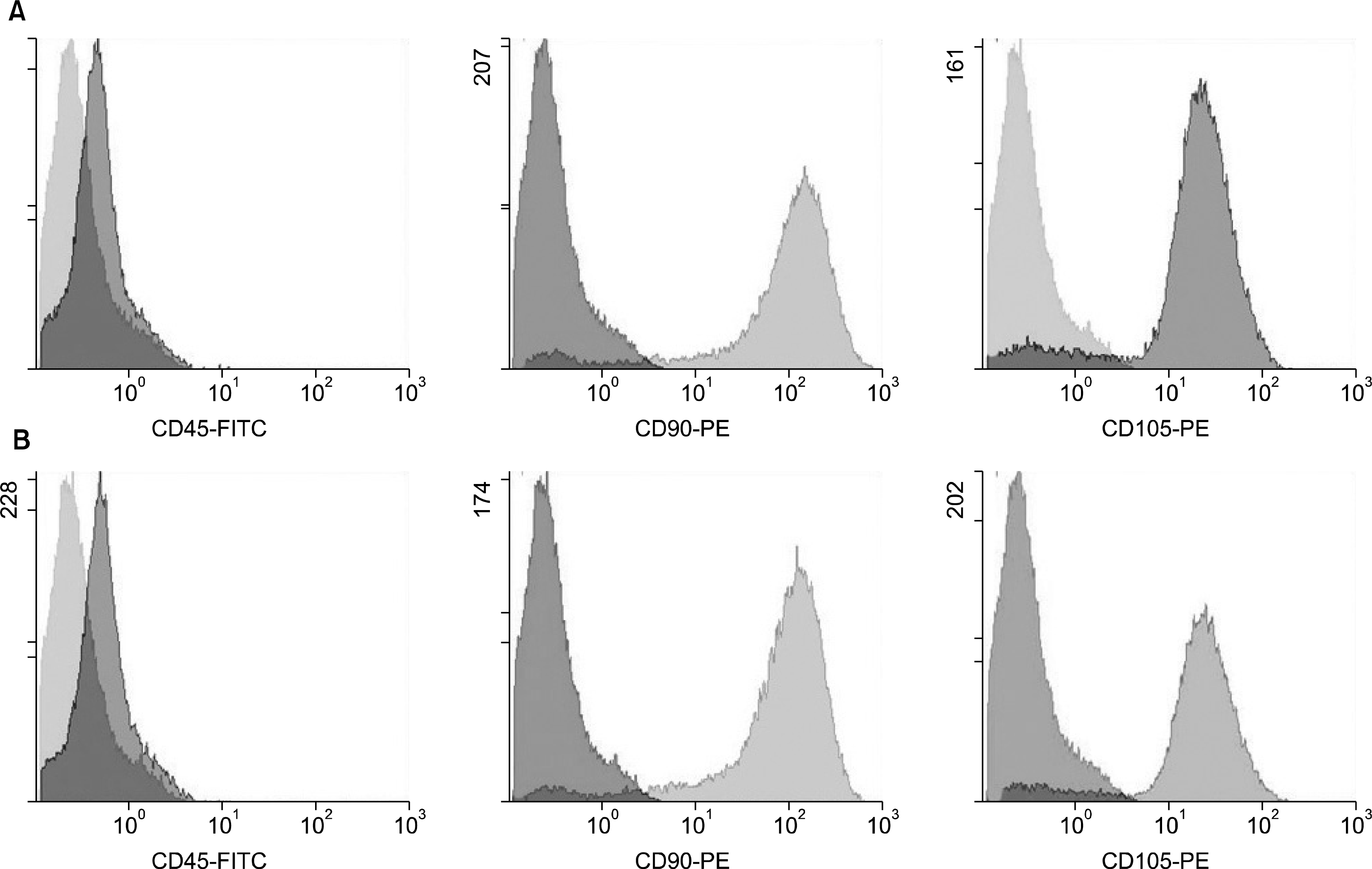 | Fig. 2.Flowcytometry analysis of hMSC. (A) Naïve hMSC isolated on day 14 after BM seeding showing negative staining for the hematopoietic marker CD45 and positive staining for the mesenchymal markers CD90 and CD105. (B) hMSC labeled with Feridex for 48 hrs. showing the same negative staining for the hematopoietic marker CD45 and positive staining for the mesenchymal markers CD90 and CD105. |
 | Fig. 3.Immunostaining analysis of naïve and Feridex-labeled hMSC. Naïve and Feridex-labeled hMSC had the classical spindle-shaped fibroblast-like morphology (A∼F). Both unlabeled and Feridex-labeled cells showed negative staining for the hematopoietic marker CD45 (A, B) and highly positive staining for CD90 (C, D) and CD105 (E, F). |
Feridex-labeled hMSC preserved their osteogenic and adipogenic differentiation potential but lost their chondrogenic differentiation property
 | Fig. 4.Mesodermal differentiation of naïve and Feridex-labeled hMSC. Naïve and labeled hMSC (A, B respectively) differentiated to osteogenic structures as shown by the NBT/BCIP (blue-purple color) staining which detects alkaline phosphatase activity. Oil-red-O staining showed that un-labeled and labeled cells (C, D respectively) formed adipocytic structures. Alcian blue staining that detects chondrogenic matrix formation showed that un-labeled hMSC formed such structures (E), whereas labeled-hMSC were unable to form chondrogenic matrix (F). |
Naïve and Feridex-labeled hMSC showed similar neural-like differentiation patterns in vitro
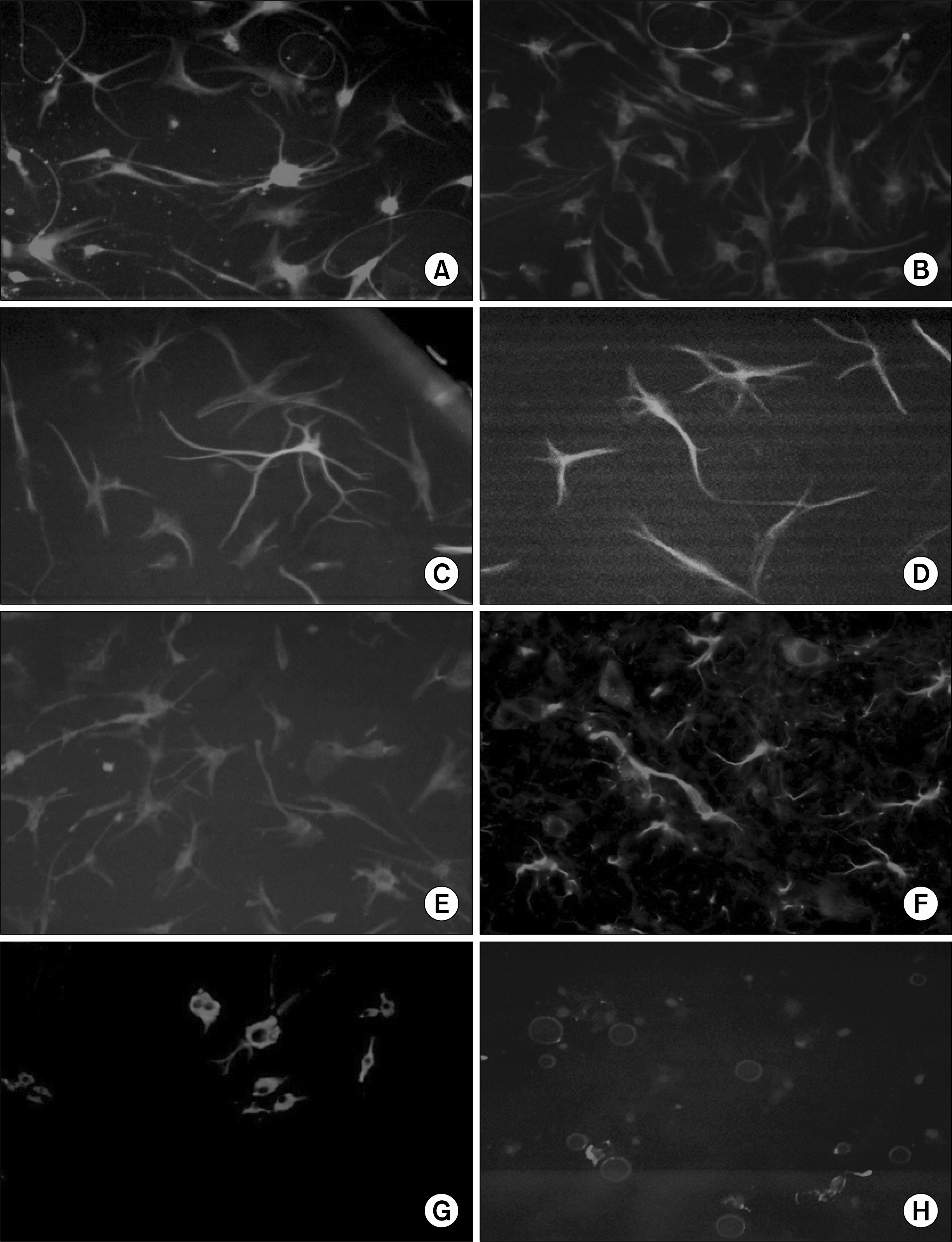 | Fig. 5.Neural differentiation of unlabeled and Feridex-labeled hMSC. Naïve and labeled cells exhibited neural-like cell morphology as seen by the positive staining for MAP2 (A, B respectively) and Tubulin-beta-III (C, D) following culture with a coktail of growth factors (as described in Methods). Astrocytic-like morphology was evaluated utilizing GFAP staining that was positive in both un-labeled cells (E) as well for the Feridex-labeled cells (F). Naïve and labeled cells showed also sparse oligodendrocytic differentiation as presented by the staining for GalC (G, H, respectively). DAPI staining was used to stain the nuclear DNA for the oligodendrocytic differentation (H). |
Feridex-labeled hMSC retain their immunomodulation properties in vitro
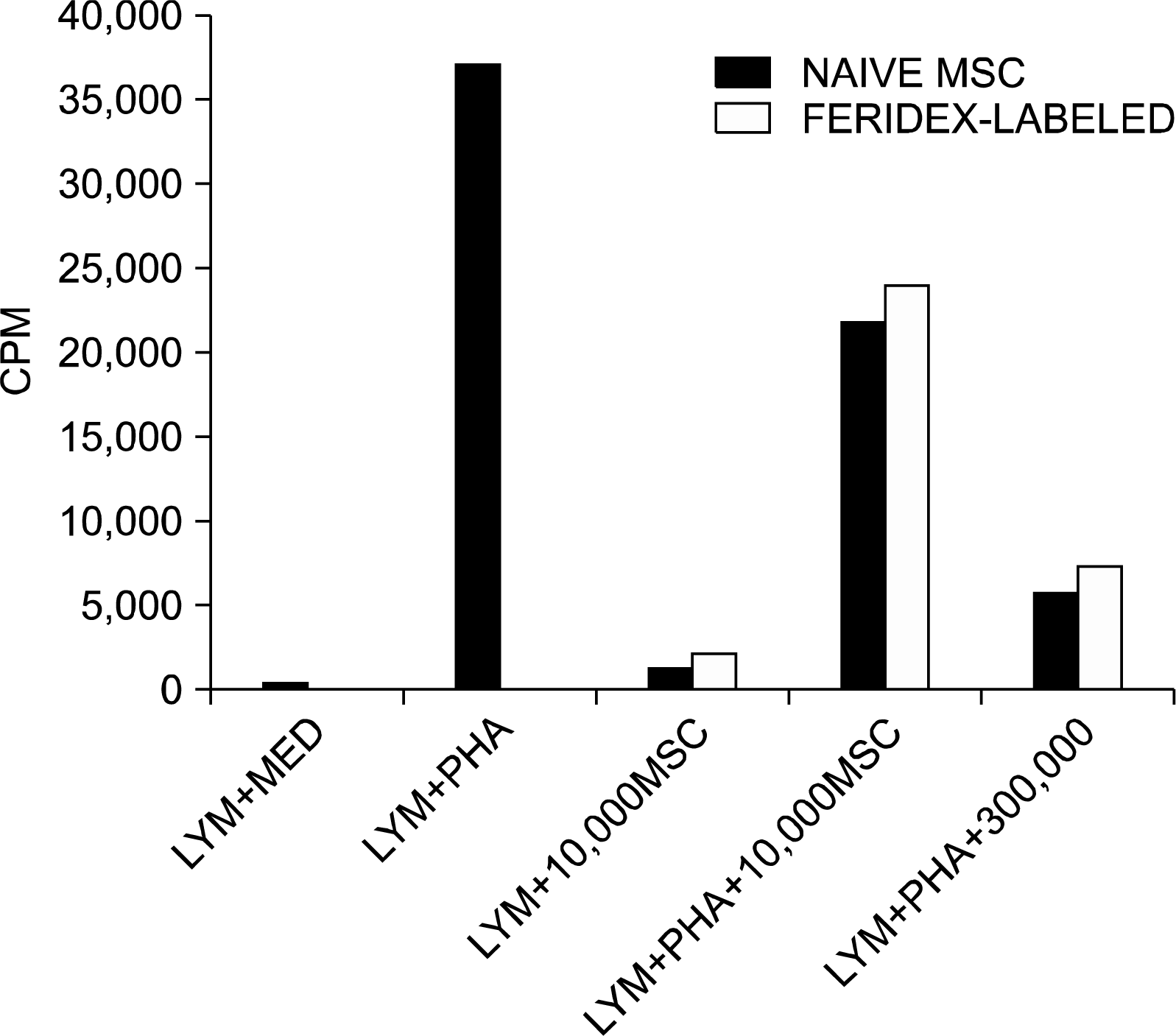 | Fig. 6.Immunomodulatory effects of unlabeled and Feridex-labeled MSCs: Effects on lymphocytic proliferation. The controls of the experiment show that lymphocytes cultured with medium or cultured with hMSC (both without the presence of PHA) did not show any proliferation effects. Lymphocytes cultured in the presence of PHA showed typical lymphocyte proliferation. Naïve and labeled hMSC were added in two doses (10×103 & 30×103) in cultures of naïve human lymphocytes, obtained from donors. Both unlabeled and Feridex-labeled hMSCs equally suppressed the proliferation of lymphocytes induced by PHA mitogen in a dose-dependent manner (MED=Medium, LYM=Lymphocytes). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download