1. Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004. 4:743–765.

2. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000. 21:2529–2543.

3. Hutmacher DW, Cool S. Concepts of scaffold-based tissue engineering--the rationale to use solid free-form fabrication techniques. J Cell Mol Med. 2007. 11:654–669.

4. Sachlos E, Czernuszka JT. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur Cell Mater. 2003. 5:29–39.

5. Cheah CM, Chua CK, Leong KF, Chua SW. Development of a tissue engineering scaffold structure library for rapid prototyping. Part 1: lnvestigation and classification. Int J Adv Manuf Technol. 2003. 21:291–301.

6. Mouriño V, Boccaccini AR. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010. 7:209–227.

7. Yeong WY, Chua CK, Leong KF, Chandrasekaran M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004. 22:643–652.

8. Leong KF, Cheah CM, Chua CK. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003. 24:2363–2378.

9. de Mulder ELW, Buma P, Hannink G. Anisotropic porous biodegradable scaffolds for musculoskeletal tissue engineering. Materials. 2009. 2:1674–1696.

10. Hutmacher DW, Sittinger M, Risbud MV. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004. 22:354–362.

11. Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005. 4:518–524.

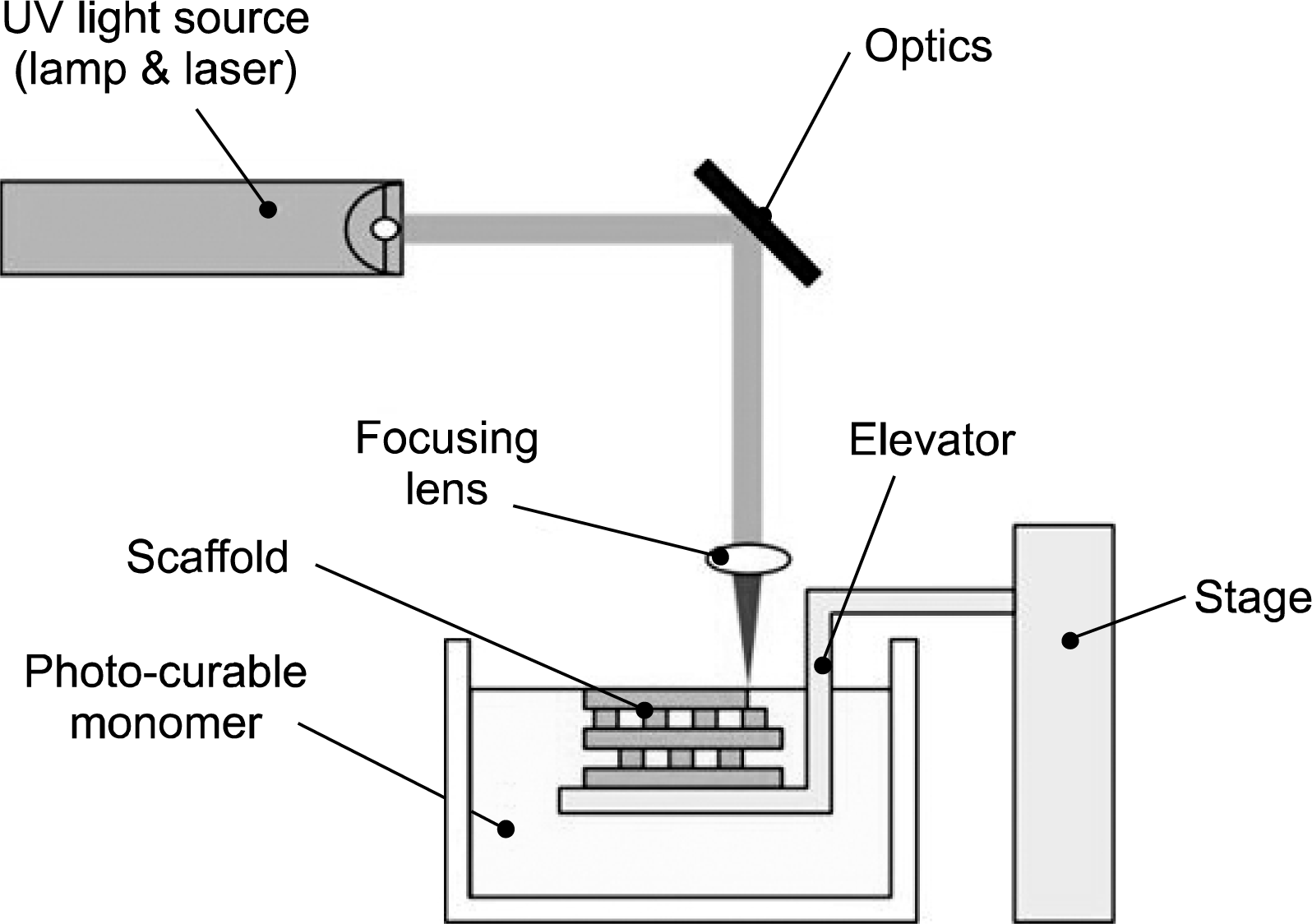
12. Melchels FP, Feijen J, Grijpma DW. A review on stereo-lithography and its applications in biomedical engineering. Biomaterials. 2010. 31:6121–6130.

13. Kodama H. Automatic method for fabricating a three-dimensional plastic model with photo-hardening polymer. Rev Sci Instrum. 1981. 52:1770–1773.

14. Nakai T, Yasuda M, Marutani Y, Ohta T. Shaping 3-D solid objects using a UV laser and photopolymer. Conference on Laser and Electro-Optics’86. 1986. ME-2:42.

15. Ikuta K, Hirowatari K, Ogata T. Three dimensional micro integrated fluid systems (MIFS). Fabricated by Stereo Lithography. Jan Oiso;Japan: 1994. 1–6.
16. Ikuta K, Maruo S, Fukaya Y, Fujisawa T. Biochemical IC chip toward cell free DNA protein synthesis. In : Annual international workshop; 11th; 1998;Jan; Heidelberg:Germany.

17. Sanderson JE. Bone replacement and repair putty material from unsaturated polyester resin and vinyl pyrrolidone. US Patent 4, 722, 948. 1988. 1–14.
18. Cooke MN, Fisher JP, Dean D, Rimnac C, Mikos AG. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B Appl Biomater. 2003. 64:65–69.

19. Lee JW, Lan PX, Kim B, Lim G, Cho DW. Fabrication and characteristic analysis of a poly (propylene fumarate) scaffold using micro-stereolithography technology. J Biomed Mater Res B Appl Biomater. 2008. 87:1–9.
20. Lee JW, Lan PX, Kim B, Lim G, Cho DW. 3D scaffold fabrication with PPF/DEF using micro-stereolithography. Microelectron Eng. 2007. 84:1702–1705.

21. Lan PX, Lee JW, Seol YJ, Cho DW. Development of 3D PPF/DEF scaffolds using micro-stereolithography and surface modification. J Mater Sci Mater Med. 2009. 20:271–279.

22. Lee KW, Wang S, Fox BC, Ritman EL, Yaszemski MJ, Lu L. Poly (propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules. 2007. 8:1077–1084.

23. Schuster M, Turecek C, Varga F, Lichtenegger H, Stampfl J, Liska R. 3D-shaping of biodegradable photopolymers for hard tissue replacement. Appl Surf Sci. 2007. 254:1131–1134.

24. Kwon IK, Matsuda T. Photo-polymerized microarchitectural constructs prepared by microstereolithography (muSL) using liquid acrylate-end-capped trimethylene carbonate-based prepolymers. Biomaterials. 2005. 26:1675–1684.

25. Lee JW, Ahn GS, Kim DS, Cho DW. Development of nano- and microscale composite 3D scaffolds using PPF/DEF-HA and micro-stereolithography. Microelectronic Eng. 2009. 86:1465–1467.

26. Lee KW, Wang S, Yaszemski MJ, Lu L. Physical properties and cellular responses to crosslinkable poly (propylene fumarate)/hydroxyapatite nanocomposites. Biomaterials. 2008. 29:2839–2848.

27. Popov VK, Evseev AV, Ivanov AL, Roginski VV, Volozhin AI, Howdle SM. Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication. J Mater Sci Mater Med. 2004. 15:123–128.

28. Chu TM, Halloran JW, Hollister SJ, Feinberg SE. Hydroxyapatite implants with designed internal architecture. J Mater Sci Mater Med. 2001. 12:471–478.
29. Woesz A, Rumpler A, Stampfl J, Varga F, Fratzl-Zelman N, Roschger P, Klaushofer K, Fratzl P. Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gelcasting. Mater Sci Eng C. 2005. 25:181–186.

30. Schuster M, Inführ R, Turecek C, Stampfl J, Varga F, Liska R. Photopolymers for rapid prototyping of soluble mold materials and molding of cellular biomaterials. Monatshefte für Chemie. 2006. 137:843–853.

31. Cabañas MV, Peña J, Román J, Vallet-Regí M. Room temperature synthesis of agarose/sol-gel glass pieces with tailored interconnected porosity. J Biomed Mater Res A. 2006. 78:508–514.

32. Sánchez-Salcedo S, Nieto A, Vallet-Regí M. Hydroxyapatite/
β-tricalcium phosphate/agarose macroporous scaffolds for bone tissue engineering. Chem Eng J. 2008. 137:62–71.

33. Kang HW, Rhie JW, Cho DW. Development of a bi-pore scaffold using indirect solid freeform fabrication based on microstereolithography technology. Microelectron Eng. 2009. 86:941–944.

34. Kang HW, Seol YJ, Cho DW. Development of an indirect solid freeform fabrication process based on microstereo-lithography for 3D porous scaffolds. J Micromech Microeng. 2009. 19:015011.

35. Jiankang H, Dichen L, Yaxiong L, Bo Y, Bingheng L, Qin L. Fabrication and characterization of chitosan/gelatin porous scaffolds with predefined internal microstructures. Polymer. 2007. 48:4578–4588.

36. Zein I, Hutmacher DW, Tan KC, Teoh SH. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002. 23:1169–1185.

37. Chim H, Hutmacher DW, Chou AM, Oliveira AL, Reis RL, Lim TC, Schantz JT. A comparative analysis of scaffold material modifications for load-bearing applications in bone tissue engineering. Int J Oral Maxillofac Surg. 2006. 35:928–934.

38. Jones AC, Milthorpe B, Averdunk H, Limaye A, Senden TJ, Sakellariou A, Sheppard AP, Sok RM, Knackstedt MA, Brandwood A, Rohner D, Hutmacher DW. Analysis of 3D bone ingrowth into polymer scaffolds via micro-computed tomography imaging. Biomaterials. 2004. 25:4947–4954.

39. Meakin JR, Shepherd DE, Hukins DW. Short communication: fused deposition models from CT scans. Br J Radiol. 2004. 77:504–507.
40. Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005. 26:3739–3748.

41. Woodfield TB, Malda J, de Wijn J, Péters F, Riesle J, van Blitterswijk CA. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004. 25:4149–4161.

42. Yamada A, Niikura F, Ikuta K. A three-dimensional micro-fabrication system for biodegradable polymers with high resolution and biocompatibility. J Micromech Microeng. 2008. 18:025035.

43. Wang F, Shor L, Darling A, Khalil S, Sun W, Güçeri S, Lau A. Precision extruding deposition and characterization of cellualr poly-
ε-caprolactone tissue scaffolds. Rapid Prototyping J. 2004. 10:42–49.

44. Kim JY, Park EK, Kim SY, Shin JW, Cho DW. Fabrication of a SFF-based three-dimensional scaffold using a precision deposition system in tissue engineering. J Micromech Microeng. 2008. 18:055027.

45. Domingos M, Dinucci D, Cometa S, Alderighi M, Bártolo PJ, Chiellini F. Polycaprolactone scaffolds fabricated via bioextrusion for tissue engineering applications. Int J Biomaterials. 2009. 2009:239643.

46. Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001. 55:203–216.

47. Choong C, Triffitt JT, Cui ZF. Polycaprolactone scaffolds for bone tissue engineering: effects of a calcium phosphate coating layer on osteogenic cells. Food Bioprod Process. 2004. 82:117–125.
48. Cao T, Ho KH, Teoh SH. Scaffold design and in vitro study of osteochondral coculture in a three-dimensional porous polycaprolactone scaffold fabricated by fused deposition modeling. Tissue Eng. 2003. 9(Suppl 1):S103–S112.
49. Kalita SJ, Ferguson M. Fabrication of 3-D porous Mg/Zn doped tricalcium phosphate bone-scaffolds via the fused deposition modelling. Am J Biochem Biotechnol. 2006. 2:57–60.

50. Shor L, Güçeri S, Chang R, Gordon J, Kang Q, Hartsock L, An Y, Sun W. Precision extruding deposition (PED) fabrication of polycaprolactone (PCL) scaffolds for bone tissue engineering. Biofabrication. 2009. 1:015003.

51. Xiong Z, Yan Y, Wang S, Zhang R, Zhang C. Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scripta Mater. 2002. 46:771–776.

52. Kim JY, Yoon JJ, Park EK, Kim DS, Kim SY, Cho DW. Cell adhesion and proliferation evaluation of SFF-based biodegradable scaffolds fabricated using a multi-head deposition system. Biofabrication. 2009. 1:015002.

53. Kim JY, Jin GZ, Park IS, Kim JN, Chun SY, Park EK, Kim SY, Yoo J, Kim SH, Rhie JW, Cho DW. Evaluation of solid free-form fabrication-based scaffolds seeded with osteoblasts and human umbilical vein endothelial cells for use in vivo osteogenesis. Tissue Eng Part A. 2010. 16:2229–2236.

54. Rimell JT, Marquis PM. Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. J Biomed Mater Res. 2000. 53:414–420.

55. Tan KH, Chua CK, Leong KF, Cheah CM, Cheang P, Abu Bakar MS, Cha SW. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003. 24:3115–3123.

56. Tan KH, Chua CK, Leong KF, Cheah CM, Gui WS, Tan WS, Wiria FE. Selective laser sintering of biocompatible polymers for applications in tissue engineering. Biomed Mater Eng. 2005. 15:113–124.
57. Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005. 26:4817–4827.

58. Kim SH, Seo BM, Choung PH, Lee YM. Adult stem cell therapy for periodontal disease. Int J Stem Cell. 2010. 3:16–21.

59. Liulan L, Qingxi H, Xianxu H, Gaochun X. Design and fabrication of bone tissue engineering scaffolds via rapid prototyping and CAD. J Rare Earth. 2007. 25:379–383.

60. Wiria FE, Leong KF, Chua CK, Liu Y. Poly-epsilon-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomater. 2007. 3:1–12.

61. Zhou WY, Lee SH, Wang M, Cheung WL, Ip WY. Selective laser sintering of porous tissue engineering scaffolds from poly (L: -lactide)/carbonated hydroxyapatite nanocomposite microspheres. J Mater Sci Mater Med. 2008. 19:2535–2540.

62. Kanczler JM, Mirmalek-Sani SH, Hanley NA, Ivanov AL, Barry JJ, Upton C, Shakesheff KM, Howdle SM, Antonov EN, Bagratashvili VN, Popov VK, Oreffo RO. Biocompatibility and osteogenic potential of human fetal femur-derived cells on surface selective laser sintered scaffolds. Acta Biomater. 2009. 5:2063–2071.

63. Lam CXF, Mo XM, Teoh SH, Hutmacher DW. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002. 20:49–56.

64. Seitz H, Rieder W, Irsen S, Leukers B, Tille C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005. 74:782–788.

65. Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003. 21:157–161.

66. Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005. 26:93–99.

67. Limpanuphap S, Derby B. Manufacture of biomaterials by a novel printing process. J Mater Sci Mater Med. 2002. 13:1163–1166.
68. Lee M, Dunn JC, Wu BM. Scaffold fabrication by indirect three-dimensional printing. Biomaterials. 2005. 26:4281–4289.

69. Mondrinos MJ, Dembzynski R, Lu L, Byrapogu VK, Wootton DM, Lelkes PI, Zhou J. Porogen-based solid free-form fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering. Biomaterials. 2006. 27:4399–4408.

70. Khalyfa A, Vogt S, Weisser J, Grimm G, Rechtenbach A, Meyer W, Schnabelrauch M. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J Mater Sci Mater Med. 2007. 18:909–916.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download