1. Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009. 94:451–454.

2. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989. 321:1174–1178.

3. Escolar ML, Poe MD, Provenzale JM, Richards KC, Allison J, Wood S, Wenger DA, Pietryga D, Wall D, Champagne M, Morse R, Krivit W, Kurtzberg J. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005. 352:2069–2081.

4. Slatter MA, Gennery AR. Umbilical cord stem cell transplantation for primary immunodeficiencies. Expert Opin Biol Ther. 2006. 6:555–565.

5. Davey S, Armitage S, Rocha V, Garnier F, Brown J, Brown CJ, Warwick R, Fehily D, Watt S, Gluckman E, Vora A, Contreras M, Navarrete CV. The London Cord Blood Bank: analysis of banking and transplantation outcome. Br J Haematol. 2004. 125:358–365.

6. Fasouliotis SJ, Schenker JG. Human umbilical cord blood banking and transplantation: a state of the art. Eur J Obstet Gynecol Reprod Biol. 2000. 90:13–25.

7. Grewal SS, Barker JN, Davies SM, Wagner JE. Unrelated donor hematopoietic cell transplantation: marrow or umbilical cord blood? Blood. 2003. 101:4233–4244.

8. Kekarainen T, Mannelin S, Laine J, Jaatinen T. Optimization of immunomagnetic separation for cord blood-derived hematopoietic stem cells. BMC Cell Biol. 2006. 730.

9. Broxmeyer HE, Gluckman E, Auerbach A, Douglas GW, Friedman H, Cooper S, Hangoc G, Kurtzberg J, Bard J, Boyse EA. Human umbilical cord blood: a clinically useful source of transplantable hematopoietic stem/progenitor cells. Int J Cell Cloning. 1990. 8(Suppl 1):76–89.

10. Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005. 23:1105–1112.

11. Denner L, Bodenburg Y, Zhao JG, Howe M, Cappo J, Tilton RG, Copland JA, Forraz N, McGuckin C, Urban R. Directed engineering of umbilical cord blood stem cells to produce C-peptide and insulin. Cell Prolif. 2007. 40:367–380.

12. McGuckin C, Forraz N, Baradez MO, Basford C, Dickinson AM, Navran S, Hartgerink JD. Embryonic-like stem cells from umbilical cord blood and potential for neural modeling. Acta Neurobiol Exp (Wars). 2006. 66:321–329.
13. McGuckin CP, Basford C, Hanger K, Habibollah S, Forraz N. Cord blood revelations-The importance of being a first born girl, big, on time and to a young mother! Early Hum Dev. 2007. 83:733–741.

14. Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, Taylor PE, Stevens CE. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995. 92:10119–10122.

15. Vannier JP, Monconduit M, Piguet H. Comparison between 2 density gradients for separation of CFU. Biomedicine. 1980. 33:236–239.
16. Solves P, Mirabet V, Planelles D, Blasco I, Perales A, Carbonell-Uberos F, Soler MA, Roig R. Red blood cell depletion with a semiautomated system or hydroxyethyl starch sedimentation for routine cord blood banking: a comparative study. Transfusion. 2005. 45:867–873.

17. Chow R, Nademanee A, Rosenthal J, Karanes C, Jaing TH, Graham ML, Tsukahara E, Wang B, Gjertson D, Tan P, Forman S, Petz LD. Analysis of hematopoietic cell transplants using plasma-depleted cord blood products that are not red blood cell reduced. Biol Blood Marrow Transplant. 2007. 13:1346–1357.

18. Ademokun JA, Chapman C, Dunn J, Lander D, Mair K, Proctor SJ, Dickinson AM. Umbilical cord blood collection and separation for haematopoietic progenitor cell banking. Bone Marrow Transplant. 1997. 19:1023–1028.

19. Berger MJ, Adams SD, Tigges BM, Sprague SL, Wang XJ, Collins DP, McKenna DH. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006. 8:480–487.

20. Lapierre V, Pellegrini N, Bardey I, Malugani C, Saas P, Garnache F, Racadot E, Schillinger F, Maddens S. Cord blood volume reduction using an automated system (Sepax) vs. a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy. 2007. 9:165–169.

21. Meyer-Monard S, Passweg J, Troeger C, Eberhard HP, Roosnek E, de Faveri GN, Chalandon Y, Rovo A, Kindler V, Irion O, Holzgreve W, Gratwohl A, Müller C, Tichelli A, Tiercy JM. Cord blood banks collect units with different HLA alleles and haplotypes to volunteer donor banks: a comparative report from Swiss Blood stem cells. Bone Marrow Transplant. 2009. 43:771–778.

22. McGuckin CF, Forraz N. Umbilical cord blood stem cells--an ethical source for regenerative medicine. Med Law. 2008. 27:147–165.
23. Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, Goldman A, Kersey J, Krivit W, MacMillan ML, Orchard PJ, Peters C, Weisdorf DJ, Ramsay NK, Davies SM. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002. 100:1611–1168.

24. Sauter C, Barker JN. Unrelated donor umbilical cord blood transplantation for the treatment of hematologic malignancies. Curr Opin Hematol. 2008. 15:568–575.

25. Lister J, Gryn JF, McQueen KL, Harris DT, Rossetti JM, Shadduck RK. Multiple unit HLA-unmatched sex-mismatched umbilical cord blood transplantation for advanced hematological malignancy. Stem Cells Dev. 2007. 16:177–186.

26. Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, de Lima M, Cairo MS, Fürst S, Rio B, Dalley C, Carreras E, Harousseau JL, Mohty M, Taveira D, Dreger P, Sureda A, Gluckman E, Rocha V. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the euro-cord-netcord and lymphoma working party of the european group for blood and marrow transplantation. J Clin Oncol. 2009. 27:256–263.

27. Van Haute I, Lootens N, De Buck K, Verdegen L, Vander Steene V, Desmet S, Craeye D, Vandekerckhove B. Selecting cord blood units for storage by CD34+ cell counts. Transfusion. 2005. 45:455–457.

28. Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999. 20:330–335.

29. Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, Lapatra S, Tort L, Sunyer JO. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006. 7:1116–1124.

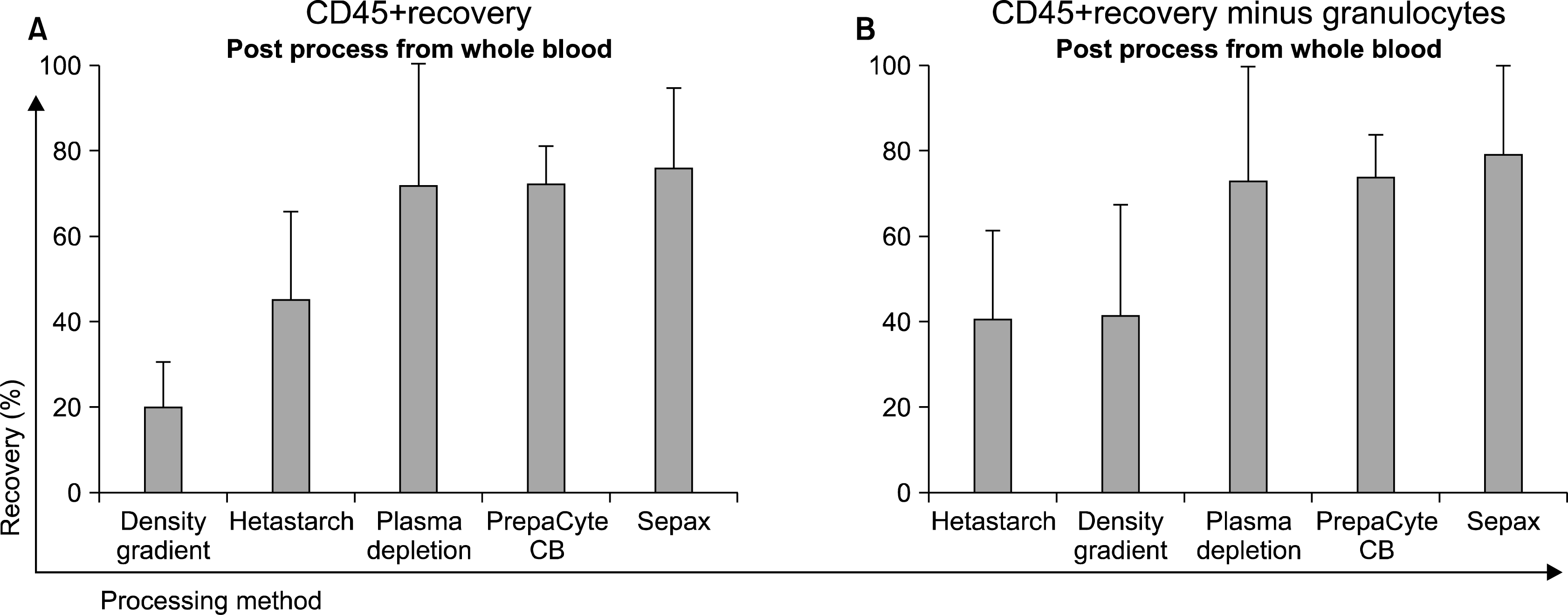
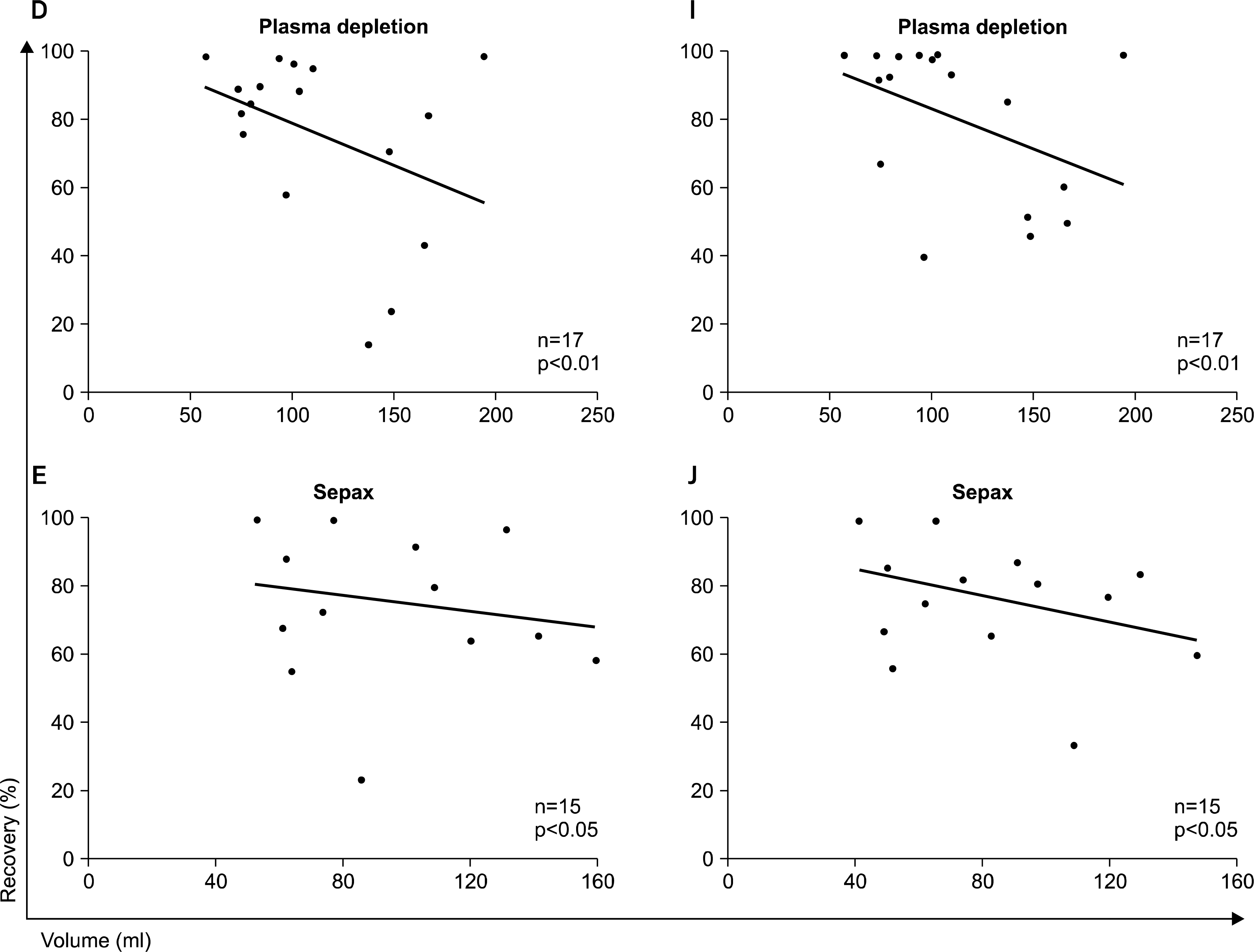
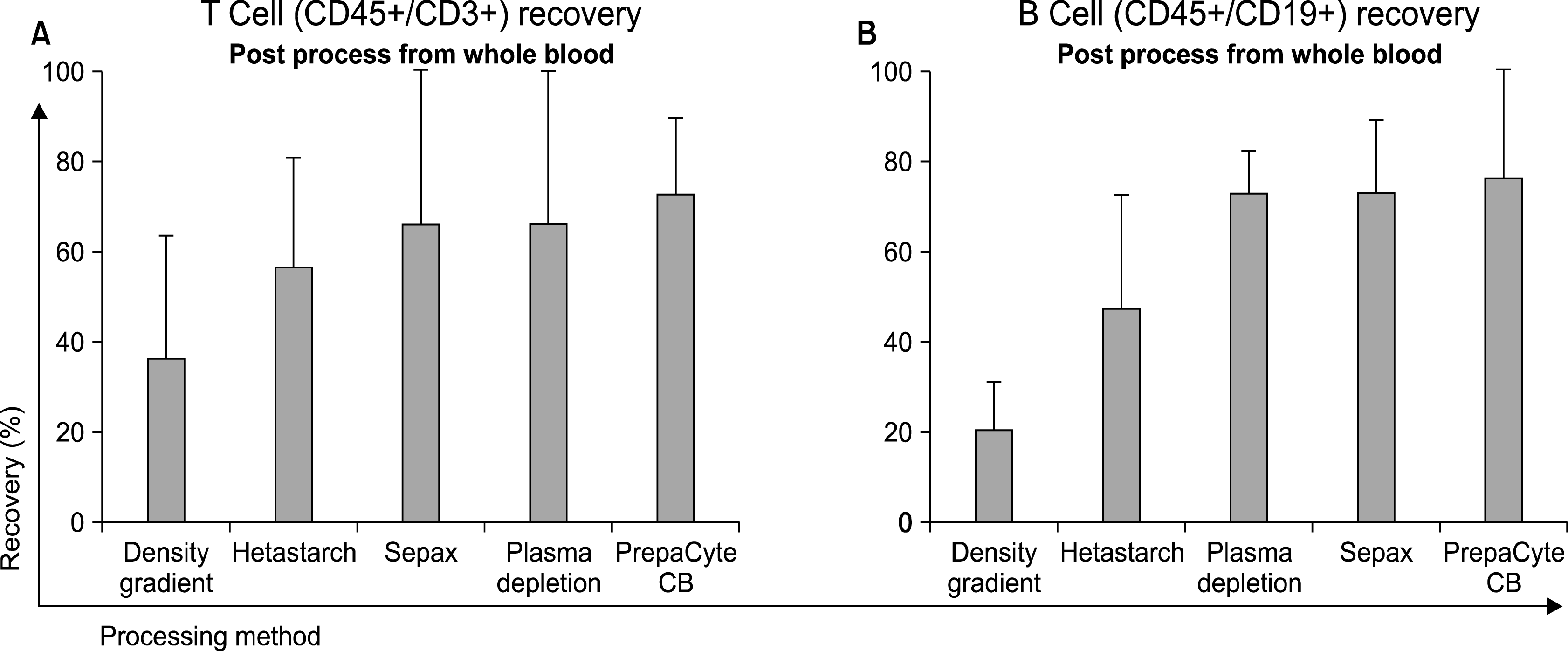
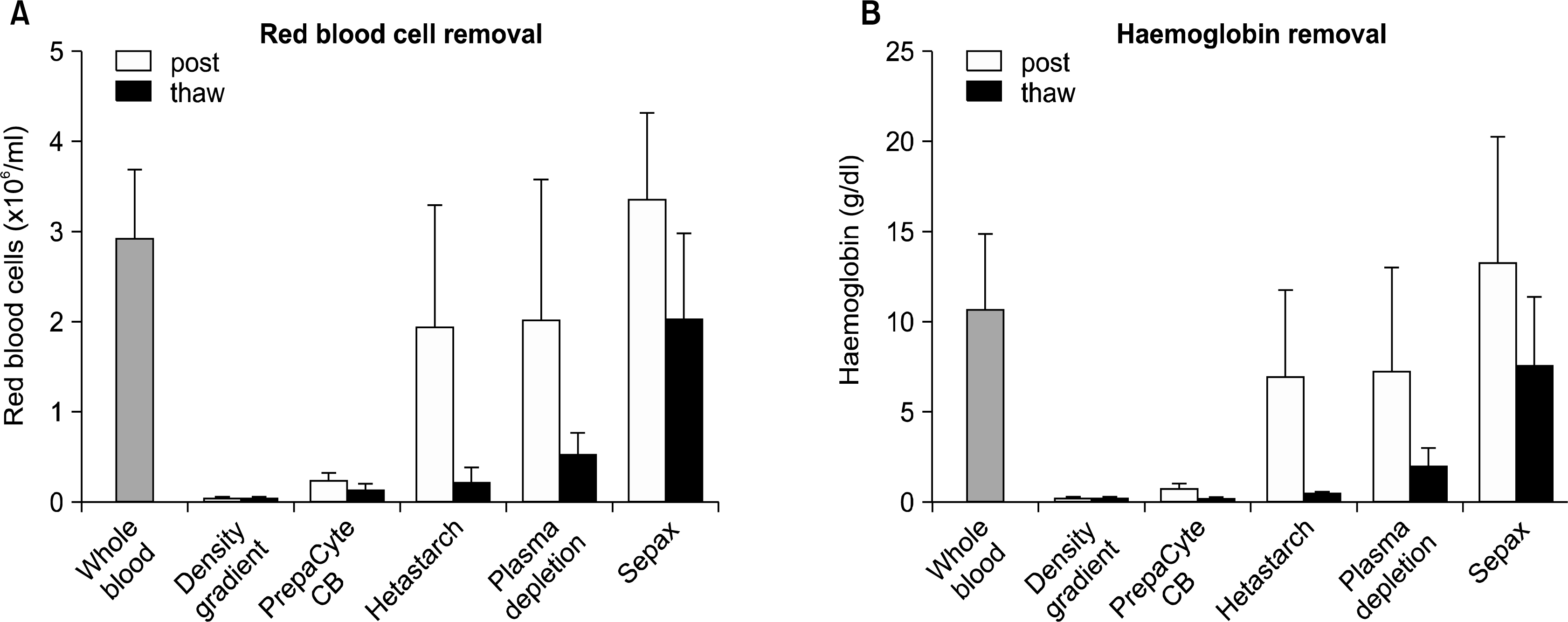
30. Laroche V, McKenna DH, Moroff G, Schierman T, Kadidlo D, McCullough J. Cell loss and recovery in umbilical cord blood processing: a comparison of postthaw and postwash samples. Transfusion. 2005. 45:1909–1916.

31. Committee on Obstetric Practice; Committee on Genetics. ACOG committee opinion number 399, February 2008: umbilical cord blood banking. Obstet Gynecol. 2008. 111:475–477.
32. Yoo KH, Lee SH, Kim HJ, Sung KW, Jung HL, Cho EJ, Park HK, Kim HA, Koo HH. The impact of post-thaw colony-forming units-granulocyte/macrophage on engraftment following unrelated cord blood transplantation in pediatric recipients. Bone Marrow Transplant. 2007. 39:515–521.

33. de Kreuk AM, Zevenbergen A, van Oostveen JW, Schuurhuis GJ, Huijgens PC, Jonkhoff AR. A single-step colony-forming unit assay for unseparated mobilized peripheral blood, cord blood, and bone marrow. J Hematother Stem Cell Res. 2001. 10:795–806.

34. Samuel GN, Kerridge IH, O’Brien TA. Umbilical cord blood banking: public good or private benefit? Med J Aust. 2008. 188:533–535.






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download