Introduction
Thromboangiitis obliterans (TAO, Buerger’s disease) is nonatherosclerotic inflammatory disease of the peripheral blood vessels, and it affects the small and medium sized vessels of the extremities (1). Felix von Winiwarter, as a student of Billroth at the University Clinic in Vienna, first described TAO in 1879 and Leo Buerger described in exquisite detail the clinical and histopathological features of the disease in 1908 (2), yet the etiology of TAO is still largely unknown. TAO predominantly occurs in young males who habitually use tobacco (3). TAO intensifies usually at the 4th decade and then the symptoms diminish. TAO is almost never observed in persons over the age of 60 years.
TAO is clinically and pathologically distinguishable from other forms of vasculitis according to some points. For the pathology, there is a highly cellular and inflammatory thrombus with relative sparing of the blood vessel wall. An immune reaction has been demonstrated in the arterial intima, yet the acute phase reactants and serologic tests for the immunologic markers and the commonly measured autoantibodies are normal or negative (4).
Go to : 
Epidemiology
TAO has a worldwide, but quite uneven distribution. It has more prevalent in the Middle East and Far East than in North America and Western Europe. It appears that TAO occurs with the least frequency in Western Europe (TAO makes up 0.5∼5.6% of the patients with peripheral arterial disease, PAD) and the most commonly afflicted are the people of India, Japan, Korea, Bangladesh and the Ashkenazi Jews in Israel (1, 5). High morbidity is observed in India (45∼63%), Korea and Japan (16∼66%) (1, 6). The reasons for this pattern are unclear. The high prevalence in some areas has been attributed to the use of specific types of tobacco (7). The question of a genetic predisposition has been raised by several investigators (8∼10).
A decline in the number of patients who are diagnosed with TAO has been observed in the developed countries. A literature has reported that the prevalence rate of diagnosing TAO in North America has declined steadily from 104/100,000 in 1947 to 13/100,000 in 1986 (an 8 fold decrease), and the clinical and pathological criteria for the diagnosis of TAO have remained unchanged (3). In Japan, the prevalence of diagnosing TAO has decreased (6). However, a dramatic increase in the incidence of female TAO has been observed. A marked increase of female TAO was reported by several studies and the increased prevalence of TAO in women may be attributed to the increase rate of smoking by young women (3, 11).
Go to : 
Pathophysiology
Although the cause of TAO is still unknown, there is an extremely strong association between the heavy use of tobacco and TAO, and what precipitates the disease and determinates its course is smoking (12, 13). It mainly affects young male smokers, although a few cases have been reported in ex-smokers and users of smokeless tobacco (14, 15). TAO is more common in countries whose people heavily use tobacco or they use unprocessed low-grade tobacco called “bidi” (a homemade surrogate for cigarettes without filters) (7, 16). Some author believe that TAO can occur in nonsmokers (17); however, most authors believe current smoking or a past smoking history is required for making the diagnosis (16, 18, 19). It has long been recognized that persistent tobacco use, and most commonly cigarette smoking, is a major risk factor for disease persistence, progression and recurrence. Clinical recurrence is almost always associated with resumption of tobacco use.
Endothelial cells play a key role in the initiation of the inflammatory response. Eichhorn et al. suggested an increased serum antiendothelial-cell antibody titer was related with the disease activity and pathogenesis of TAO (20). Halacheva et al. described an increased expression of adhesive molecules such as VCAM-1, ICAM-1 and selectin on the surface of endothelial cell from patients with TAO (21). In TAO patients, the cellular sensitivity to types I and III collagen were increased on an antigen-sensitive thymidine-incorporation assay (22). Endothelial dysfunction is reflected by impaired endothelium dependent vasorelaxation in the peripheral vasculature of patients with TAO, as was observed in studies that focused on the forearm blood flow (23).
Several studies have been conducted to determine the immunologic and genetic factors related with TAO. A multifactorial etiology of TAO may involve the interplay between hereditary susceptibility, tobacco exposure and the immune and coagulation responses.
Go to : 
Clinical features
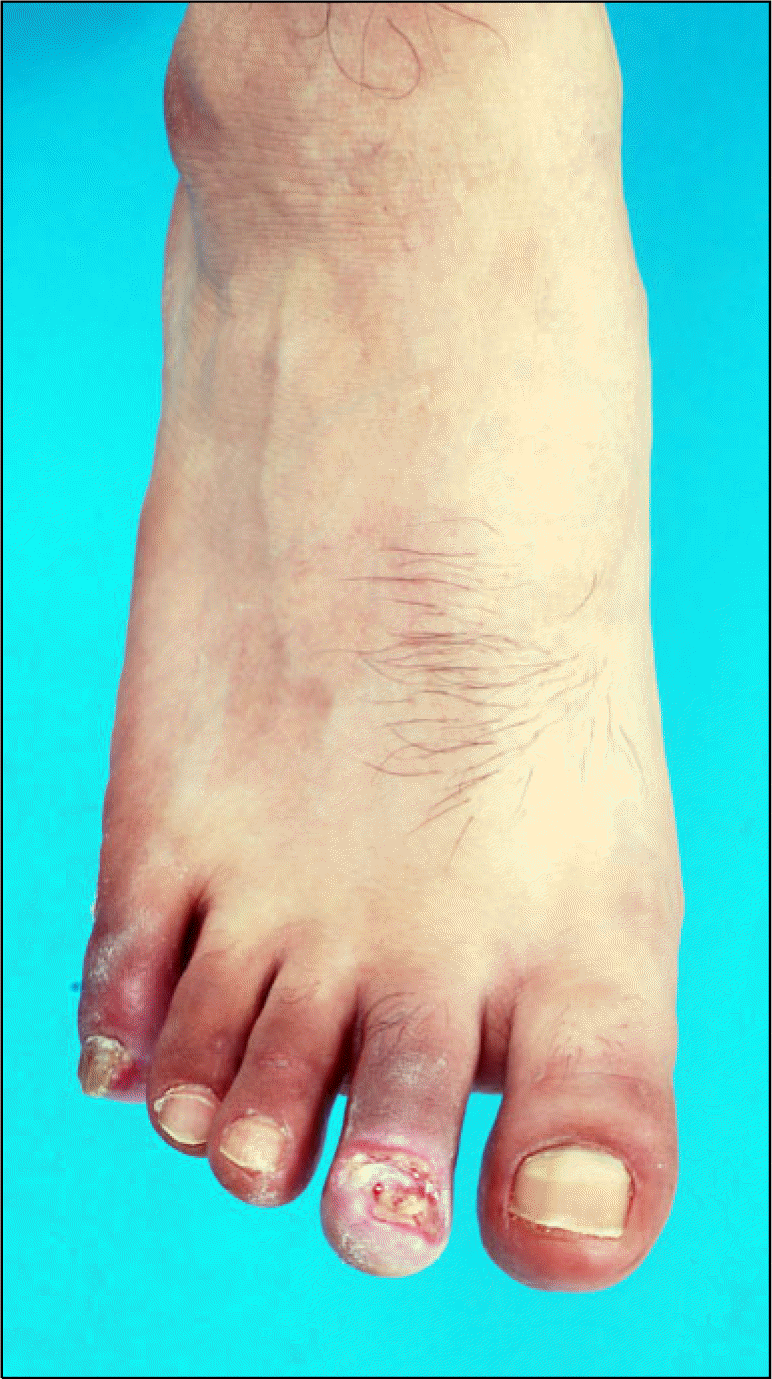
Chronic ischemia in the lower extremities is mainly caused by arterial obstruction or stenosis in the leg. TAO usually begins with ischemia of the distal small arteries and veins of the extremities. The first clinical manifestation the patients notices is coldness, skin color changes, intermittent claudication, pain or ulceration (Fig. 1). Claudication is a much less common complaint for patients with TAO than for patients with atherosclerosis. Whereas the clinical features show staged progression in the arterial insufficiency of atherosclerosis, in that of TAO, rest pain or ulceration does not always follow claudication (13). Ischemic rest pain and ulceration of the forefoot are the most frequently encountered clinical presentations. Superficial thrombophlebitis can be observed in the patients with TAO and it is a distinguishing clinical feature (1, 24). The frequent upper extremity involvement, which is characterized by the development of Raynaud’s syndrome or digital ischemia, differs from the patients with atherosclerosis (25). Skin color change is characteristic of TAO. The affected peripheral extremity is abnormally red and cyanotic particularly on dependency. The cause of the stagnation of the peripheral circulation might be poor inflow, multiple occlusion of peripheral arteries and veins, or atony of the microcirculatory vessels.
The disease rarely affects the vessels proximal to the popliteal artery. However, the aortoiliac region is sporadically affected and involvement of the mesenteric artery, cerebral artery, coronary artery and renal artery during the course of the TAO has been described in the literature (26–30).
Go to : 
Diagnosis
The diagnosis can often be made on the basis of a careful history and physical examination, together with the ancillary laboratory studies. Several diagnostic criateria have been suggested, but any commonly accepted diagnostic criteria are still unavailable. Mills and Poster (31) proposed major and minor diagnostic criteria and Papa et al. (32) proposed a scoring system based on the negative and positive criteria. The clinical criteria suggested by Shionoya in 1998 are some of the most recently used criteria. Shionoya’s criteria for making the diagnosis of TAO is based on 5 criteria (a smoking history, an onset before the age of 50 years, infrapopliteal arterial occlusive disease, either upper limb involvement or phlebitis migrans, and the absence of atherosclerotic risk factors other than smoking). As there is no specific diagnostic test and an absence of positive serologic markers, a confident clinical diagnosis should be made only when all these 5 criteria have been fulfilled, although this is not universally accepted (13, 19).
Because of the likelihood of involvement of more than one limb, it is advisable to evaluate all four limbs in patients who present with clinical involvement of only one limb. Noninvasive vascular testing, laboratory tests to exclude hypercoagulable states autoimmune disease and diabetes mellitus, and echocardiography and arteriography for exclusion of the proximal source of emboli are mandatory.
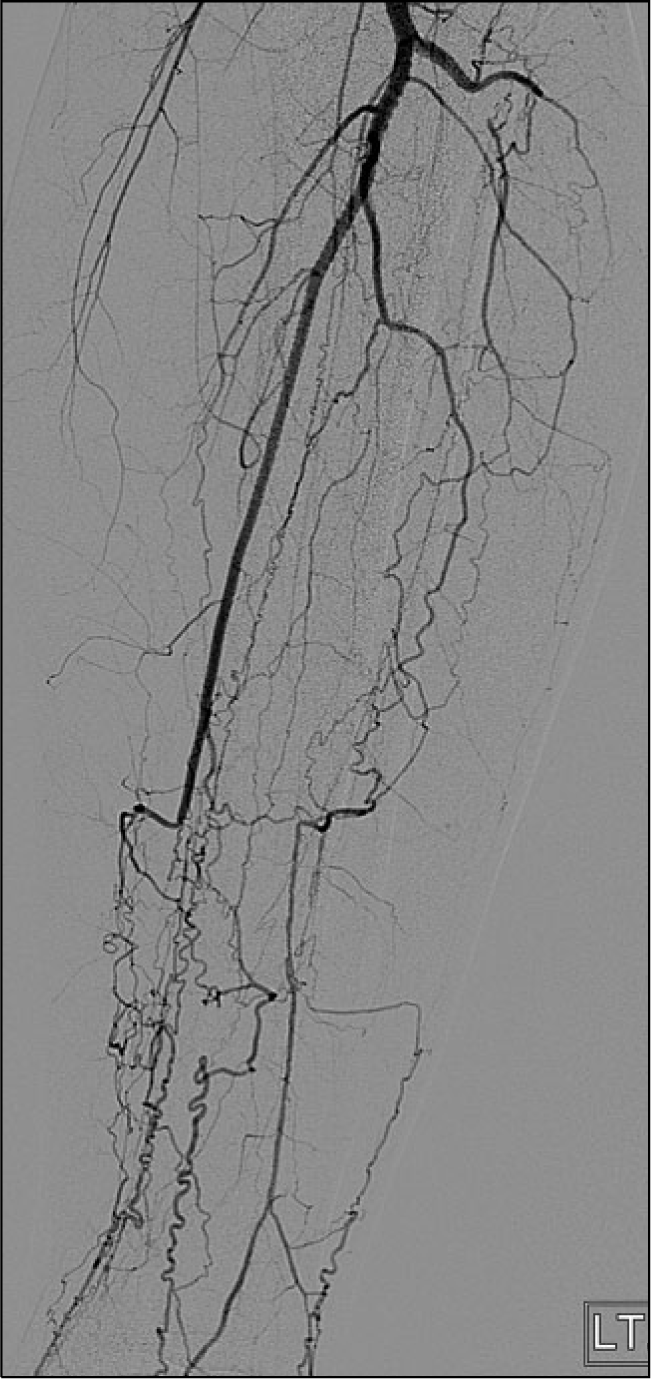
Computed tomographic angiography or conventional arteriography is useful for evaluating the clinically involved and noninvolved limbs. The angiographic findings in patients with TAO typically involve the medium and small sized arteries that are localized distal to the elbow and/or the knee. An abrupt occlusion, skip lesions and segmental lesions can be noticed and the characteristic “corkscrew,” “spider legs” or “tree roots” are helpful, but these are not pathognomonic (33) (Fig. 2).
Go to : 
Treatment
Successful therapy is possible only with absolute abstinence from tobacco. Tobacco consumption, in any form, must be strictly prohibited. A wide spectrum of medical or surgical therapeutic options have been proposed, yet total abstinence from tobacco use remains the most important mean of stopping disease progression (34). Continued tobacco use is associated with a multiplied rate of amputation (35). The initial management of patients with TAO should be conservative. Because several arteries may be unaffected, patients with claudication should be encouraged to walk, whereas the patients with critical ischemia should be admitted to a hospital for bed rest. A mild reverse Trendelenburg position may be helpful and administration of narcotic analgesics may be required. Infected lesions should be treated with appropriate antibiotics and limited debridement as required to control infection. Administration of a prostacyclin analogue that has vasodilatory and antiplatelet properties may be helpful to relieve the ischemic symptoms in the TAO patients (36, 37).
The role of sympathectomy in preventing amputation and for treating painful or nonhealing ischemic lesions remains unclear (25, 38). Sympathectomy may occasionally help the healing of superficial ischemic ulceration. It may be employed in a patient with persistent severe pain and minor ischemic lesions despite abstaining from tobacco.
A spinal cord stimulator showed benefit in the treatment of the ischemic extremity lesions of TAO, and this resulted in healing of ulcerations and a good limb survival rate (39, 40). However, there is currently no prospective randomized evidence that spinal cord stimulation is effective in healing ischemic lesions.
In TAO, concern should be focused on healing trophic lesion and salvaging the affected limbs. Major amputation of the extremity must be avoided if possible, and necrotomy will be recommended after the boundary between the living and necrotic tissue has been well defined.
Bypass grafting is seldom an option as the distal location of the lesions leaves little to bypass because of the lack of distal target vessels and the poor vein quality due to previous phlebitis. Although direct arterial surgery is usually not feasible, successful arterial revascularization is most effective for healing trophic lesion. A literature review revealed only a few series that reported on vascular reconstruction (mainly femorodistal bypasses) in patients with TAO (13). The bypass patency rates were suboptimal; however, the corresponding limb salvage rates were satisfactory. A possible explanation for this is that patent grafts, even over a short period of time, are sufficient to allow healing of ulcers in patients with TAO (41, 42). The toe blood pressure did not return to normal after successful femoraodistal bypass grafting because of multiple arterial occlusions below the ankle, and improvement of inflow did not bring about a significant increase in the toe blood pressure.
The prognosis for patients with TAO is significantly worse with respect to limb loss than that for the patients with atherosclerosis (24). It has been reported that TAO is not associated with increased mortality and the prognosis is considerably better than that for patients with atherosclerosis (13, 24). Yet a recent study observed that the survival of TAO patients was lower than that in the general population and the risk of death was nearly identical for those TAO subjects who continued to use tobacco and those who quit (34).
Go to : 
Application of stem cell therapy
Surgical bypass or endovascular (transluminal angioplasty or intravascular stent) therapy is currently believed to be the best option for limbs salvage in the eligible patients. However, these treatments are usually not possible for patients with TAO because of the diffuse segmental involvement and the distal nature of the disease. Therefore, novel therapeutic modalities are needed for treating patients with TAO, and especially those patients who are not eligible for conventional revascularization therapies.
The development of collateral vessels in an important physiological adaptation to chronic ischemia that is due to occlusive arterial disease, and the extent of collateralization in patients with peripheral artery disease can have a major impact on the symptoms, distal blood flow and lower limb outcomes (43). With the recent advances in molecular biology, gene therapy and stem cell therapy for the treatment of many diseases, a new and promising approach using stem cell therapy has recently been developed to treat the intractable symptoms related to ischemia in the subjects with peripheral artery disease, including TAO and atherosclerosis (44–46). New applications of biotechnology can stimulate new vessel formation via the local administration of proangiogenic growth factors in the form of recombinant protein, gene therapy or by the implantation of stem cells that will synthesize multiple angiogenic cytokines.
Formation of new vessels involves at least 3 distinct biological processes (47). Angiogenesis refers to a process in which preexisting capillaries sprout and proliferate to form networks that consist of vessels at the capillary level and this is triggered by endothelial cell activation, migration and proliferation followed by remodeling and expansion of the extracellular matrix (48). Vasculogenesis is in-situ formation of new blood vessels from circulating bone marrow-derived endothelial progenitor cells. At last, arteriogenesis refers to an increase in the wall thickness and luminal diameter of existing arteriolar collateral vessels via recruitment of perivascular cells and smooth muscle cells. New vessel formation in the lower limbs of patients with peripheral artery disease is likely to involve a combination of these three processes.
There are several methods of stem cell therapy such as intramuscular injection of bone marrow derived mono-nuclear cells, intramuscular injection of cytokine mobilized peripheral blood mononuclear cells, intramuscular injection of whole bone marrow and mobilization alone (46).
Tateishi et al. first reported the significant clinical benefits of injecting bone marrow mononuclear cells (BMMNCs) in the calf muscle in terms of the walking time, the ankle brachial index and the transcutaneous oxygen concentration (49). Several small, nonrandomized studies have replicated these results. Higashi et al. suggested that improvement of the endothelial dysfunction with BMMNCs implantation was a potential mechanism for improving limb ischemia (50).
Several authors have performed randomized trials to assess implantation of peripheral blood mononuclear cells (PBMNCs) that were mobilized by granulocyte colony stimulating factor (G-CSF) and they reported significant improvements in the ankle brachial index, the Doppler flow and the angiographic scores (45, 51–53).
Kim et al. reported the favorable results of autologous whole bone marrow transplantation in animal experiments and in the clinical trials that included patients with TAO (54, 55). They suggested that transplantation of autologous whole bone marrow is a simple, safe and effective means of inducing therapeutic angiogenesis.
Arai et al. examined the effect of injecting G-CSF into patients with intractable PAD symptoms. The patients were randomly assigned into 3 groups: a group treated with conventional drug therapy, a group treated with conventional drug therapy plus bone marrow transplantation (BMT) and a group treated with conventional drug therapy plus subcutaneous injection of G-CSF once daily for 10 days. One month after treatment, the subjective symptoms significantly improved in the G-CSF and BMT groups. The ankle-brachial pressure index and the trans-cutaneous oxygen pressure significantly increased in the BMT and G-CSF groups, but no such improvements were seen in the group that received conventional therapy alone (56). This is the least invasive mode of cell therapy for treating PAD, yet advanced PAD make it less likely for a large number of mobilized cells to be delivered to ischemic tissue due to the reduced blood flow.
The trials performed to date suggest that stem cell therapy could serve as a much needed novel therapeutic modality for the treatment of PAD. But trials of stem cell therapy for the PAD need to discriminate the etiologic factors. Especially, the patients with PAD caused by TAO, and these patients are rarely candidates for surgical treatment, will be good candidate for treatment with stem cell therapy. Several studies that are currently ongoing are focused on the treatment of PAD that is caused by TAO, and the treatments involve stem cell therapy (55, 57–62). So far, these studies have shown good treatment results and the treatments were safe for the patients. However, these studies have enrolled small numbers of subjects, they are often non-randomized studies and they are largely being performed to investigate the feasibility and safety of these approaches. For the clinical application of stem cell therapy to the TAO patient, more clinical evidence has to be gathered from appropriately designed, adequately powered trials with robust endpoint measurement.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download