1. Levesque JP, Winkler IG. Mobilization of hematopoietic stem cells: state of the art. Curr Opin Organ Transplant. 2008. 13:53–58.

2. MacMillan ML, Davies SM, Nelson GO, Chitphakdithai P, Confer DL, King RJ, Kernan NA. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National marrow donor program. Biol Blood Marrow Transplant. 2008. 14:16–22.

3. Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008. 15:285–292.

4. Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007. 39:577–588.

5. Meisel R, Laws HJ, Balzer S, Bernbeck B, Kramm C, Schönberger S, Sinha K, Tröger A, Schmitz M, Fischer J, Göbel U, Enczmann J, Dilloo D. Comparable long-term survival after bone marrow versus peripheral blood progenitor cell transplantation from matched unrelated donors in children with hematologic malignancies. Biol Blood Marrow Transplant. 2007. 13:1338–1345.

6. Luo XH, Chang YJ, Xu LP, Liu DH, Liu KY, Huang XJ. The impact of graft composition on clinical outcomes in un-manipulated HLA-mismatched/haploidentical hematopoietic SCT. Bone Marrow Transplant. 2009. 43:29–36.

7. Dey BR, Shaffer J, Yee AJ, McAfee S, Caron M, Power K, Ting DT, Colby C, Preffer F, Ballen K, Attar E, Saidman S, Tarbell N, Sachs D, Sykes M, Spitzer TR. Comparison of outcomes after transplantation of peripheral blood stem cells versus bone marrow following an identical nonmyeloablative conditioning regimen. Bone Marrow Transplant. 2007. 40:19–27.

8. Vela-Ojeda J, Garcia-Ruiz Esparza MA, Reyes-Maldonado E, Jiménez-Zamudio L, Garcia-Latorre E, Moreno-Lafont M, Estrada-Garcia I, Montiel-Cervantes L, Tripp-Villanueva F, Ayala-Sánchez M, Garcia-León LD, Borbolla- Escoboza JR, Mayani H. Clinical relevance of NK, NKT, and dendritic cell dose in patients receiving G-CSF-mobilized peripheral blood allogeneic stem cell transplantation. Ann Hematol. 2006. 85:113–120.

9. Dome B, Dobos J, Tovari J, Paku S, Kovacs G, Ostoros G, Timar J. Circulating bone marrow-derived endothelial progenitor cells: characterization, mobilization, and therapeutic considerations in malignant disease. Cytometry A. 2008. 73:186–193.

10. Lévesque JP, Winkler IG, Larsen SR, Rasko JE. Mobilization of bone marrow-derived progenitors. Handb Exp Pharmacol. 2007. 3–36.

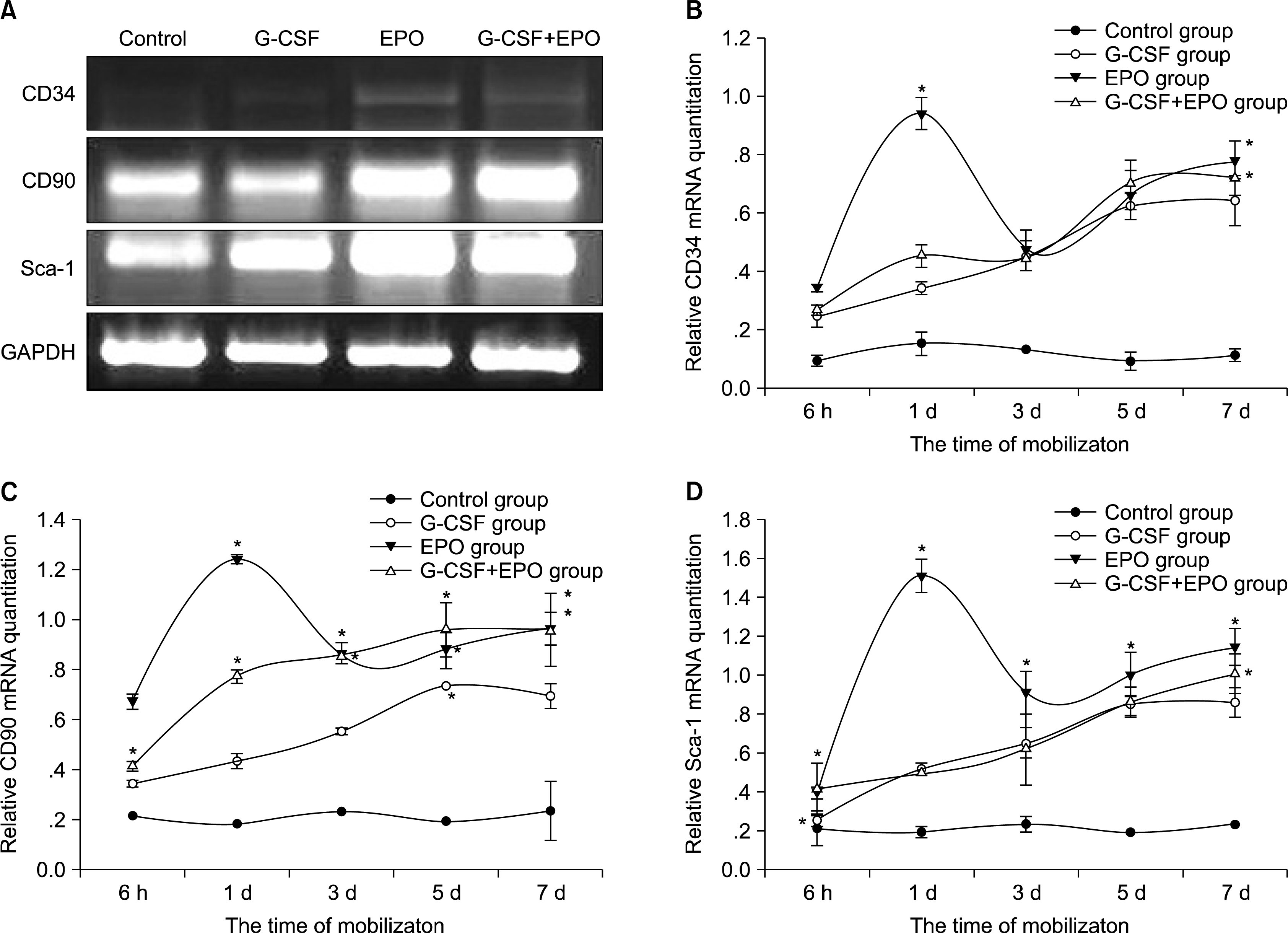
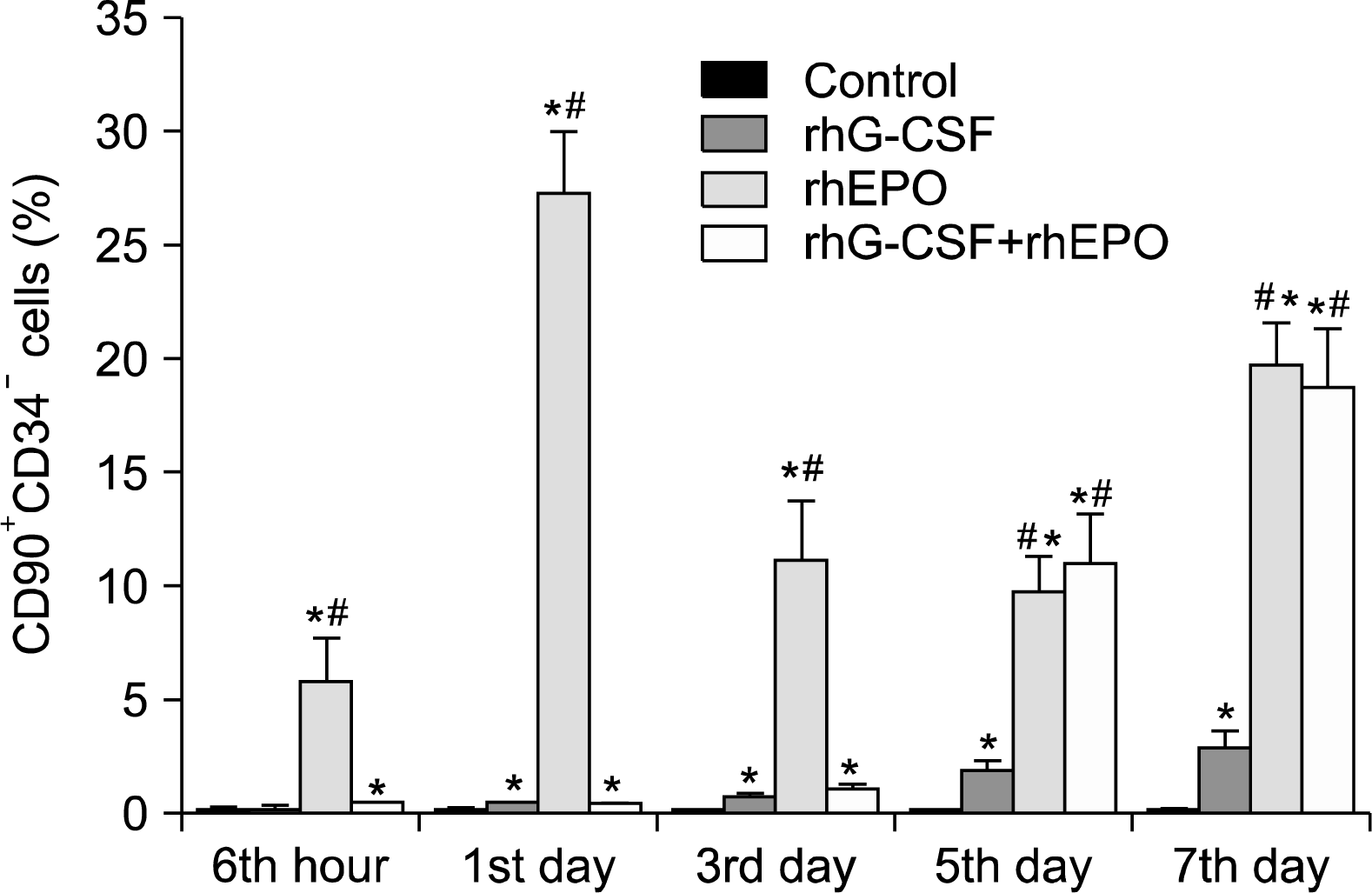
11. Grassinger J, Mueller G, Hart C, Nilsson SK, Haylock DN, Andreesen R, Hennemann B. Detection and quantification of functionally defined hematopoietic progenitor cells and tissue specific mRNA within the peripheral blood of myeloma patients after administration of granulocyte colony-stimulating factor and erythropoietin. Eur J Haematol. 2008. 80:20–30.

12. Liu B, Yang M, Li X, Qian X, Shen Z, Ding Y, Yu L. Enhanced efficiency of thermally targeted taxanes delivery in a human xenograft model of gastric cancer. J Pharm Sci. 2008. 97:3170–3181.

13. Kang HJ, Kim HS. G-CSF- and erythropoietin-based cell therapy: a promising strategy for angiomyogenesis in myocardial infarction. Expert Rev Cardiovasc Ther. 2008. 6:703–713.

14. Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008. 15:293–300.

15. Matsuoka S, Ebihara Y, Xu M, Ishii T, Sugiyama D, Yoshino H, Ueda T, Manabe A, Tanaka R, Ikeda Y, Nakahata T, Tsuji K. CD34 expression on long-term re-populating hematopoietic stem cells changes during developmental stages. Blood. 2001. 97:419–425.

16. Brunner S, Zaruba MM, Huber B, David R, Vallaster M, Assmann G, Mueller-Hoecker J, Franz WM. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008. 36:1157–1166.

17. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.

18. Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, Miyachi E, Marunouchi T, Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007. 48:43–52.

19. Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006. 37:967–976.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download