Abstract
Adipose tissue (AT) is an alternative source of the adult stem cells that can also be harvested from bone marrow (BM). Cultured AT-derived stem cells (ASCs) have been well characterized by many groups. However, non-cultured ASCs remain to be characterized. Hoechst 33342 dye efflux is a characteristic that is common to stem cells, as well as chemotherapy-resistant cancer cells. Thus, we compared the Hoechst 33342-stained side population (SP) cells in murine adipose-tissue (AT-SP cells) to the SP cells from murine bone marrow (BM-SP cells). The AT-SP cells were detected much more frequently in the 22 AT samples that were tested (0.42∼6.00%, mean 2.57%) than the BM-SP cells were detected in the 6 BM samples (0.02∼0.36%, mean 0.12%). After Hoechst staining, SP cells were analyzed by fluorescence-activated cell sorting (FACS) and electron micrograms. FACS analysis revealed that the AT-SP cells were CD44−, CD45−, CD45R+, Sca-1± and c-kit−, while the BM-SP cells were CD44−, CD45±, CD45R−, Sca-1+ and c-kit+. This indicates that the AT-SP cells differ phenotypically from the BM-SP cells. Electron microscopic analysis revealed that the AT-SP cells are small cells with a diameter of about 5 um. Some of the BM-SP cells had granules, similar to eosinophils or basophils, whereas the AT-SP cells had fewer organelles and a higher N/C ratio than the BM-SP cells. This suggests that the AT-SP cells are considerably more immature than the BM-SP cells. Thus, it appears that AT is a better source of immature non-hematopoietic cells than BM.
Adipose tissue (AT) is an alternative source of the adult stem cells that can also be harvested from bone marrow (BM) (1), skin (2), and skeletal muscle (3). However, in contrast to the latter sources, subcutaneous adipose depots are readily accessible, abundant and replenishable (4. AT-derived stem cells (ASCs) have been well characterized by many groups (4–6). CD34 is expressed by approximately 60% of non-cultured adipose-tissue stromal cells, but its expression decreases upon cell culture (4). In contrast, it appears that the frequencies of cells expressing CD13, CD29, CD44, CD73, and CD90 increase after cell culture, with over 90% of passage 4 cultured cells expressing these markers (4). ASCs do not express the hematopoietic markers CD14 and CD45 (4–6). Cultured murine ASCs express a similar profile of cell surface markers, namely, they are positive for CD29, CD44, CD105 and Sca-1, and negative for CD34 and CD45 (7). However, non-cultured ASCs from both humans and mice remain to be characterized.
Hoechst 33342 dye efflux is a characteristic that is common to stem cells, as well as chemotherapy-resistant cancer cells. It is believed that the Hoechst 33342-stained side population (SP) cells isolated from BM are more likely to be hematopoietic stem cells than the other cell populations in BM (8). Moreover, it has been reported that the SP cell populations from other tissues, such as heart, skeletal muscle, lung, skin and cornea, also contain a high frequency of stem cells (9–13). Here, we compared the SP cells in murine AT (AT-SP cells) to the SP cells from murine BM (BM-SP cells). We observed that AT-SP cells were Sca-1±, CD45- and c-kit-, while BM-SP cells were Sca-1+, CD45± and c-kit+, which is consistent with the report showing that BM-SP cells are frequently hematopoietic stem cells (14). Thus, it appears that the main population of AT-SP cells differs from hematopoietic stem cells.
The experimental procedures employed were approved by the Nippon Medical School Animal Care and Use Committee (approval number 17–25). BM-SP and AT-SP cells were prepared as described previously with some modifications (15,16). Briefly, the inguinal fat pads harvested from 6∼7 week-old C57Bl/6 mice (n=22) were mechanically minced and digested with 0.01% collagenase (Wako, Osaka, Japan) for 30 min at 37°C. After inactivation of the collagenase, the cell mixture was centrifuged at 260×g for 5 min and the cell pellet was used for analysis. The BM cells were flushed out of the femurs of 6 mice by using a 23-gauge needle. After centrifugation at 260×g for 5 min, the cell pellet was used for analysis.
The AT and BM suspensions were stained with Hoechst 33342 as described previously (17). Briefly, both suspensions were diluted in Hank’s Balanced Salt Solution (HBSS) medium to 4×105 or 1×106 cells/ml, respectively, and stained by 5 ug/ml Hoechst 33342 (Sigma-Aldrich, MO) for 90 minutes at 37°C in the presence or absence of 100 uM verapamil (Wako). Verapamil was added 10 minutes before the Hoechst dye was added.
After Hoechst staining, the cells were stained on ice for 30 minutes with fluorochrome-conjugated monoclonal rat anti-mouse antibodies (1:100) specific for Sca-1, c-kit and CD44 (BD Biosciences Pharmingen, CA), and CD45 and CD45R (Beckman Coulter, CA). 2 ug/ml of propidium iodide (Sigma) was added before FACS to exclude dead cells. The cells were analyzed and sorted by using Epics Altra (Beckman Coulter). After confirming that the addition of verapamil to the cell suspensions led to the absence of cells in the SP gating region, 1×105 and 1×104 cells of each BM and AT sample were analyzed as described previously (18) to identify the SP region.
After FACS analysis, 1×104 BM-SP and 5×104 AT-SP cells were collected, rinsed with PBS (Gibco), fixed with 2.5% glutaraldehyde, and postfixed with 1% osmium tetroxide. The Cytospin method (1,200 rpm, 10 min) was used to collect the cells in the center of the slide glass, after which the cells were dehydrated in ethanol and embedded in resin. Ultrathin sections were stained with uranyl acetate and lead citrate.
Populations of cells showing distinct low Hoechst 33342 blue/red fluorescence were detected (Fig. 1A, C). When the cell suspensions were treated with the calcium channel blocker verapamil (which prevents dye efflux), the SP cells were no longer seen (Fig. 1B, D). The AT-SP cells were detected much more frequently in the 22 AT samples that were tested (0.42∼6.00%, mean 2.57%) than the BM-SP cells were detected in the 6 BM samples (0.02∼0.36%, mean 0.12%).
Electron microscopic analysis revealed that the AT-SP cells are small cells with a diameter of about 5 um (Fig. 3A). Some of the BM-SP cells had granules, similar to eosinophils or basophils (Fig. 3B), whereas the AT-SP cells had fewer organelles and a higher N/C ratio than the BM-SP cells, and their morphology was relatively uniform (Fig. 3A). This suggests that the AT-SP cells are considerably more immature than the BM-SP cells.
This study shows for the first time that murine AT contains 20-fold more SP cells (0.42∼6.00%, mean 2.57%) than murine BM (0.02∼0.36%, mean 0.12%). The cell surface markers of the BM-SP cells were consistent with their previously reported profiles, which indicated these cells are frequently hematopoietic stem cells (18–19). However, the AT-SP cells were negative for CD45 and c-kit, therefore differing from the BM-SP cell population. Moreover, electron microscopy revealed that the AT-SP cells were more immature than the BM-SP cells. Thus, it appears that AT is a better source of immature non-hematopoietic cells than BM.
Andersen et al. (20), found that when CD45− AT-SP cells are cocultured with myoblasts in vitro, the cells exhibit myogenic differentiation. Furthermore, when non-cultured CD45− AT-SP cells are intramuscularly engrafted immediately after isolation, they generate myofibres and cells lining blood vessels (20). Moreover, it has been reported that the CD45− AT-SP cell population is enriched for cells expressing Angiopoietin 2, Ve-cadherin, endoglin, Flk1, Cd31, Cd106, and Cd133; all of these transcripts are associated with cells of the vasculature (21). This suggests that AT-SP cells may mainly be vascular progenitors and do not include as many hematopoietic stem cells as BM-SP cells.
That AT-SP cells may be vascular progenitors is of significance when seeking to determine the relationship between AT-SP cells and the so-called ASCs. It is notable that Zannetino et al. (22), described a multipotential stem cell population within adult human AT that appears to be intimately associated with perivascular cells surrounding blood vessels. Thus, it may be that AT-SP cells are mainly perivascular cells and may be the so-called ASCs. Moreover, the reason why so many SP cells exist in AT, compared to in BM, may relate to the rich vascularization of AT. Further research into this issue and the plasticity and proliferative ability of the AT-SP cells is needed.
References
2. Jones PH, Harper S, Watt FM. Stem cell patterning and fate in human epidermis. Cell. 1995. 80:83–93.

4. Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem. Cells. 2006. 24:376–385.

5. Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005. 23:412–423.

6. Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J. Cell Physiol. 2006. 208:64–76.

7. Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008. 40:2649–2654.

8. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996. 183:1797–1806.

9. Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiaion. Circ Res. 2005. 97:52–61.

10. Liadaki K, Kho AT, Sanoudou D, Schienda J, Flint A, Beggs AH, Kohane IS, Kunkel LM. Side population cells isolated from different tissues share transcriptome signatures and express tissue-specific markers. Exp Cell Res. 2005. 303:360–374.

11. Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005. 23:1073–1081.

12. Yano S, Ito Y, Fujimoto M, Hamazaki TS, Tamaki K, Okochi H. Characterization and localization of side population cells in mouse skin. Stem Cells. 2005. 23:834–841.

13. Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006. 24:86–94.

14. Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter in an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002. 99:507–512.

15. Ogawa R, Mizuno H, Watanabe A, Migita N, Shimada T, Hyakusoku H. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 2004. 313:871–878.

16. Fujimura J, Ogawa R, Mizuno H, Fukanaga Y, Suzuki H. Neural differentiation of adipose-derived stem cells isolated from GFP transgenic mice. Biochem Biophys Res Commun. 2005. 333:116–121.

17. Goodell MA. Stem cell identification and sorting using the Hoechst 33342 side population (SP). Current Protocols in Cytometry. Robinson JP, Darzynkiewicz Z, Dobrucki J, editors. unit 9.18.1 - 9.1811. John Wiley & Sons, Inc;New Jersey: 2002.

18. Pearce DJ, Ridler CM, Simpson C, Bonnet D. Multiparameter analysis of murine bone marrow side population cells. Blood. 2004. 103:2541–2546.

19. Trowbridge IS, Thomas ML. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994. 12:85–116.

20. Andersen DC, Schrøder HD, Jensen CH. Non-cultured adipose-derived CD45− side population cells are enriched for progenitors that give rise to myofibres in vivo. Exp Cell Res. 2008. 314:2951–2964.

Fig. 1.
Hoechst 33342 staining - A: AT cells, B: verapamil-treated AT cells, C: BM cells, D: verapamil-treated BM cells. The figure shows Hoechst 33342-stained cells that were harvested from one donor. In the 1×104 AT (A) and 1×105 BM (C) cell populations, approximately 4% and 0.02% were SP cells, respectively. The SP cells disappeared upon verapamil treatment (B, D). The AT-SP cells were much more frequent in the 22 AT samples (0.42∼6.00%, mean 2.57%) than the BM-AT cells in the 6 BM samples (0.02∼0.36%, mean 0.12%).
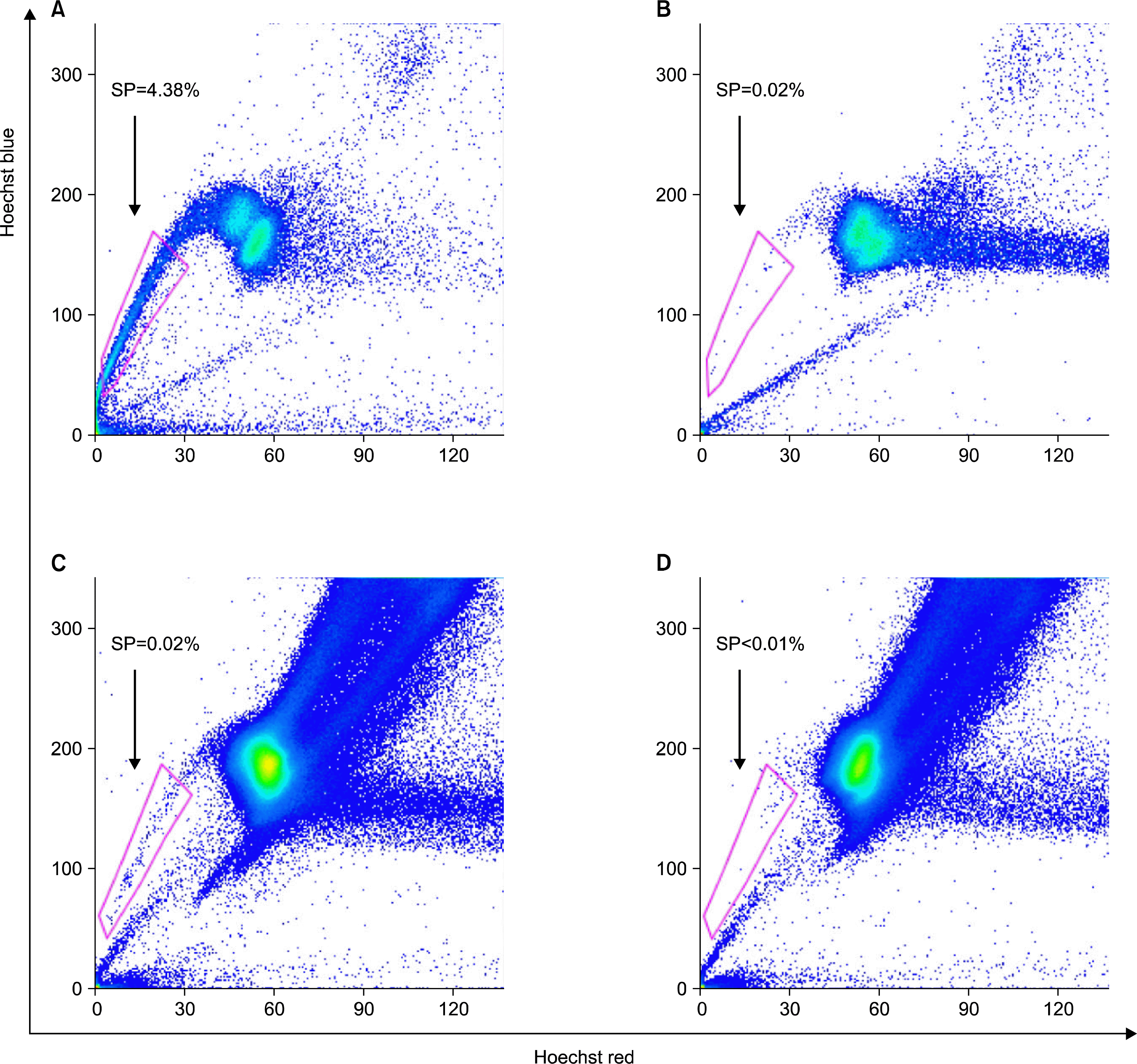
Fig. 2.
FACS analysis of SP cells - A: AT-SP cells, B: BM-SP cells. The AT-SP cells in the 22 AT samples were generally CD44−, CD45−, CD45R+, Sca-1± and c-kit− (A), while the BM-SP cells in the 6 BM samples were generally CD44−, CD45±, CD45R−, Sca-1+ and c-kit+ (B).
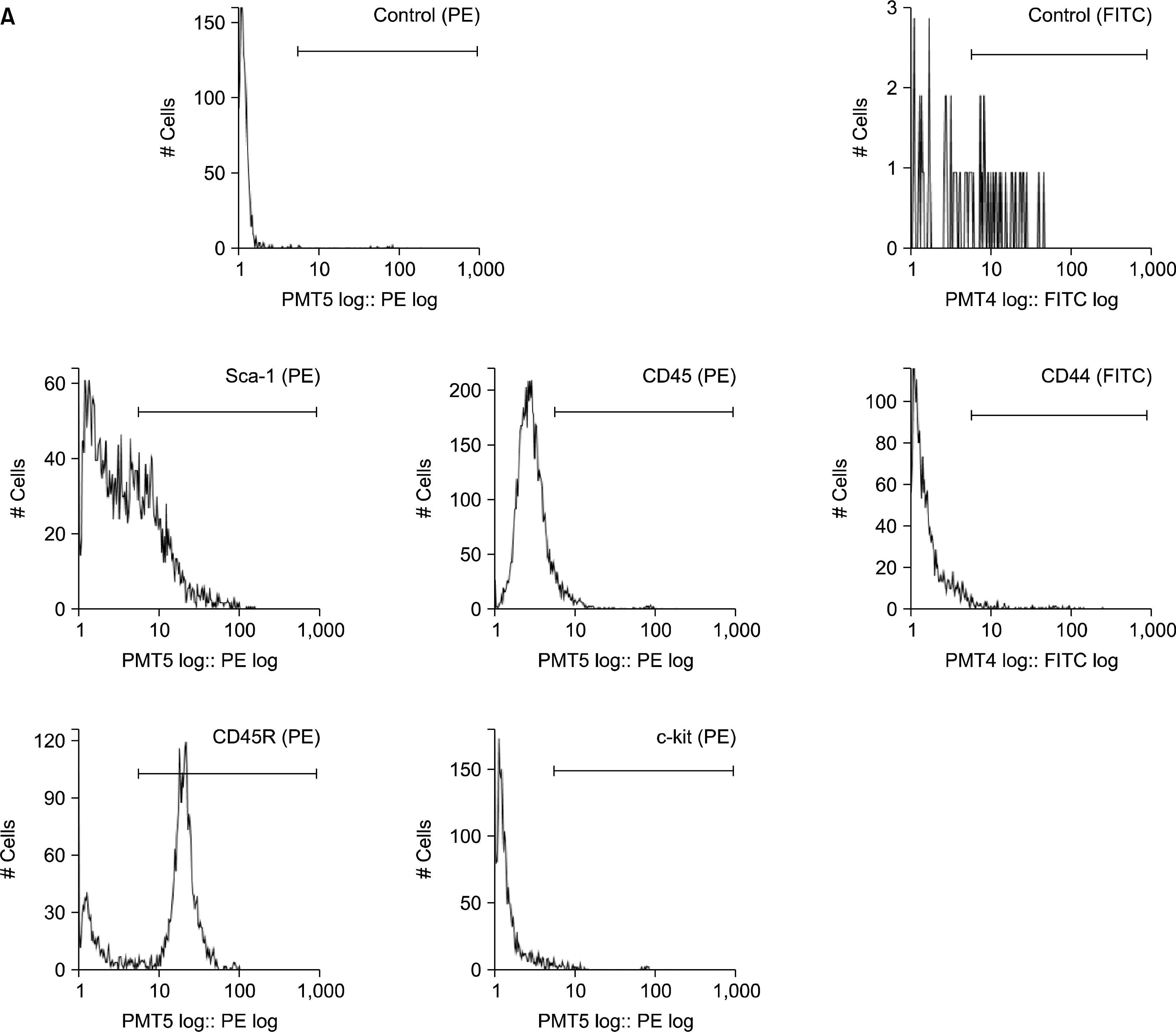
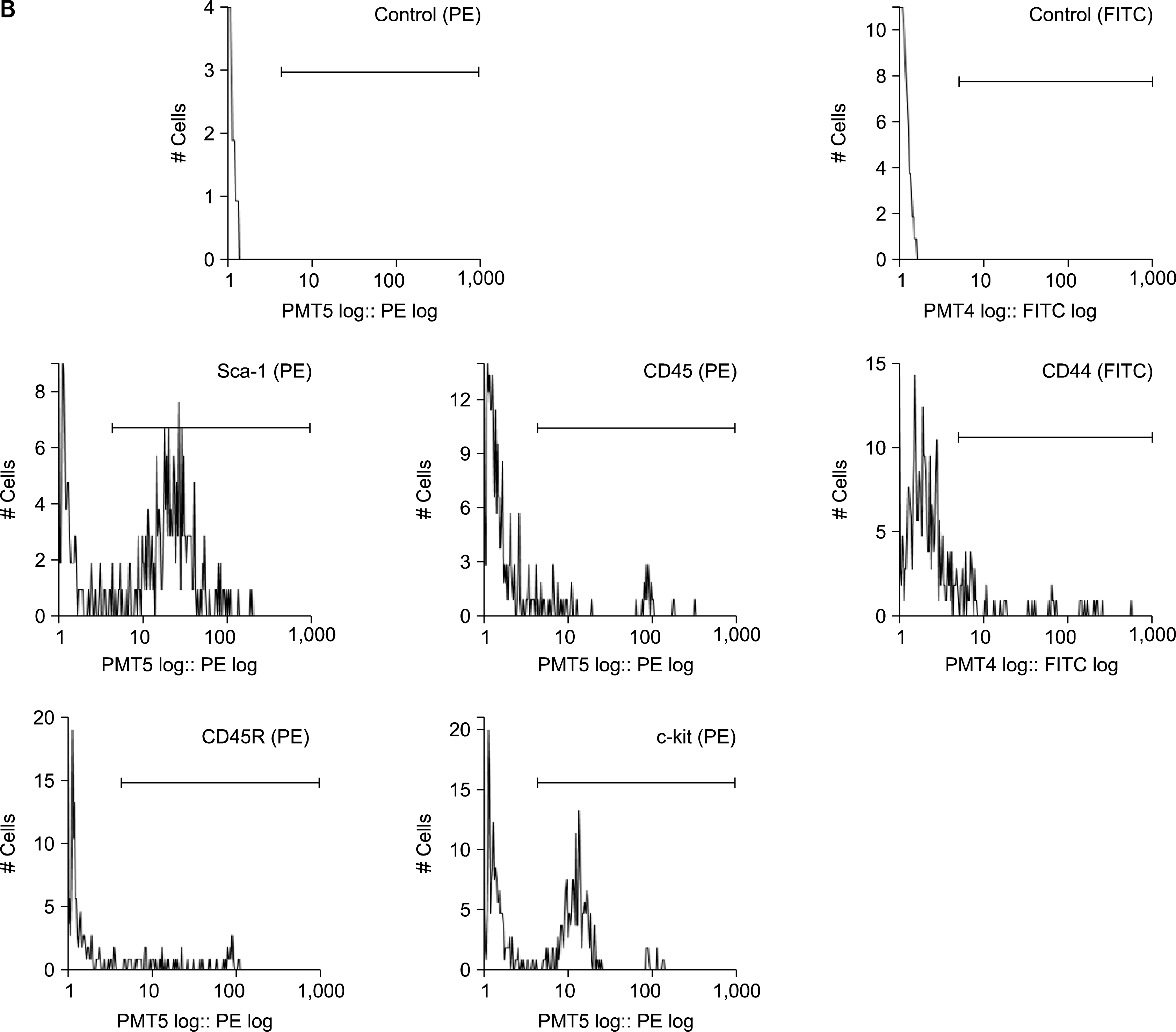
Fig. 3.
Electron micrographs. (A) AT-SP cells, (B) BM-SP cells. Electron microscopic analysis indicated that the AT-SP cells are small cells with a diameter of about 5 um (A). Some of the BM-SP cells have granules, similar to eosinophils or basophils (B), while the AT-SP cells have fewer organelles and a higher N/C ratio than the BM-SP cells, and their morphology is relatively uniform (A). This suggests that the AT-SP cells are considerably more immature than the BM-SP cells.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download