Abstract
Background and Objectives
Numerous in vitro observations have been published to show that mature adipocytes may resume proliferation and begin to populate the adipofibroblast fraction or form other cell types.
Methods and Results:
In the present study, we evaluated clonal cultures of mature pig-derived adipocytes as they began to reestablish their ability to divide. The lipid contained within the cytoplasm was either moved to the apical ends of the cell, or large droplets were physically extruded from the cell. In the latter case, we ascertained that the cell lipid droplet was handled in a different manner to that by beef-derived adipocytes as described in other published studies.
Conclusions:
Pig-derived adipocytes expel large amounts of lipid directly into the medium environment prior to becoming capable of cell division, rather than retaining all lipids like the beef cells. This difference in lipid handling and trafficking may be a novel mechanism in adipocyte resumption of proliferation.
Adipogenesis has been defined as the overall outcome from the prenatal and postnatal processes of proliferation, differentiation, and maturation of stem-like (mesodermal) cells to form terminally committed cells of the fat cell lineage, which are capable of conducting lipogenesis and the subsequent storage of visible triacylglycerides (1–5). This understanding of adipogenesis is primarily based upon the use of rodent cell lines or stromal vascular cells and is somewhat flawed, unidirectional and inflexible (1, 4–8). For example, there is little room in the definition for any other cell physiology. In other words, once the cell begins to accumulate lipid and express markers of “differentiation”, the cell is terminally differentiated (reviewed in (4)). Any adipose depot hyperplasia from that point onward is via either spindle-shaped preadipocytes or other stem cells (without lipid) that reside in the depot (1, 2). We suggest that mechanisms to regulate adipose deposition patterns (within all animals) should also include one additional mechanism, the regulation of the mature adipocyte-derived progeny (purified) adipofibroblasts (1, 3–5, 8–22) from different adipose depots.
A historical perspective of the evolution of research in this area, as well as thoughts regarding physiological options for the cell phenotype converting to a proliferative competent cell, have been presented previously (3–5, 7, 8). While once purely speculation, a groundswell of support now exists to suggest that mature adipocytes possess the ability to resume the proliferative program and, even with lipid, can begin to divide (6, 13, 23, 24).
What happens to the progeny cells that result from such dedifferentiation? Are these cells committed to the adipose lineage, or were they ever committed to a single phenotype? Non-clonal cultures of mature adipocytes (11, 16, 25) have suggested that proliferative-competent progeny cells may produce other forms of stem cells (26), while data derived from clonal (purified) cultures (3) of proliferative-capable mature adipocytes have suggested that similar possibilities may exist (6, 24).
We have provided information about the prevalence of mature cells in adipose tissue that possess ability to undergo dedifferentiation, which is a significant number, and we have defined specific methods to isolate and propagate clonal cultures of these cells (3). Our studies have shown that purified cultures (3) of mature adipocytes behave quite differently than batch cultures of mature adipocytes. First, the time required for mature adipocyte dedifferentiation is substantially longer than that observed in non-clonal cultures, even if exposed to similar in vitro conditions (3, 24). Second, proliferation dynamics (24) and handling of the lipid load appears to be different depending on the animal species from which the mature adipocytes were obtained. For example, clonal cultures of beef-derived mature adipocytes resume proliferation in both a symmetric (equal amounts of parental cell lipid retained by both progeny cells) or an asymmetric (unequal amounts of lipid retained by both progeny cells) manner (3–5). In none of the cultures examined, did the beef-derived adipocyte expel lipid from the cell. Moreover, in the purified bovine-derived system, the progeny adipocytes appear to be proliferative-competent (4, 24) but undergo redifferentiation in a different manner (24) and also express some differences in intrinsic programming than other adipogenesis cell systems (27, 28).
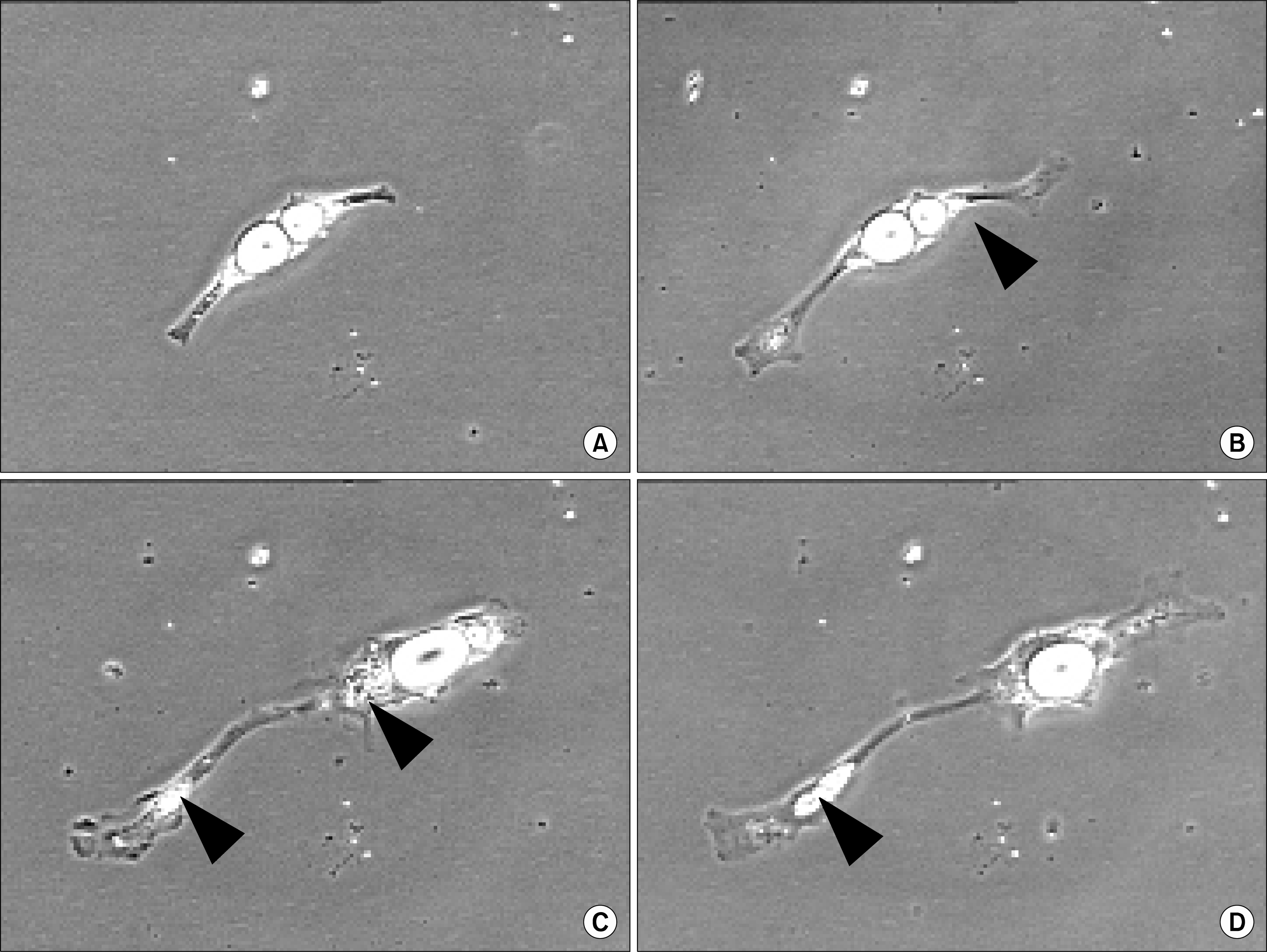
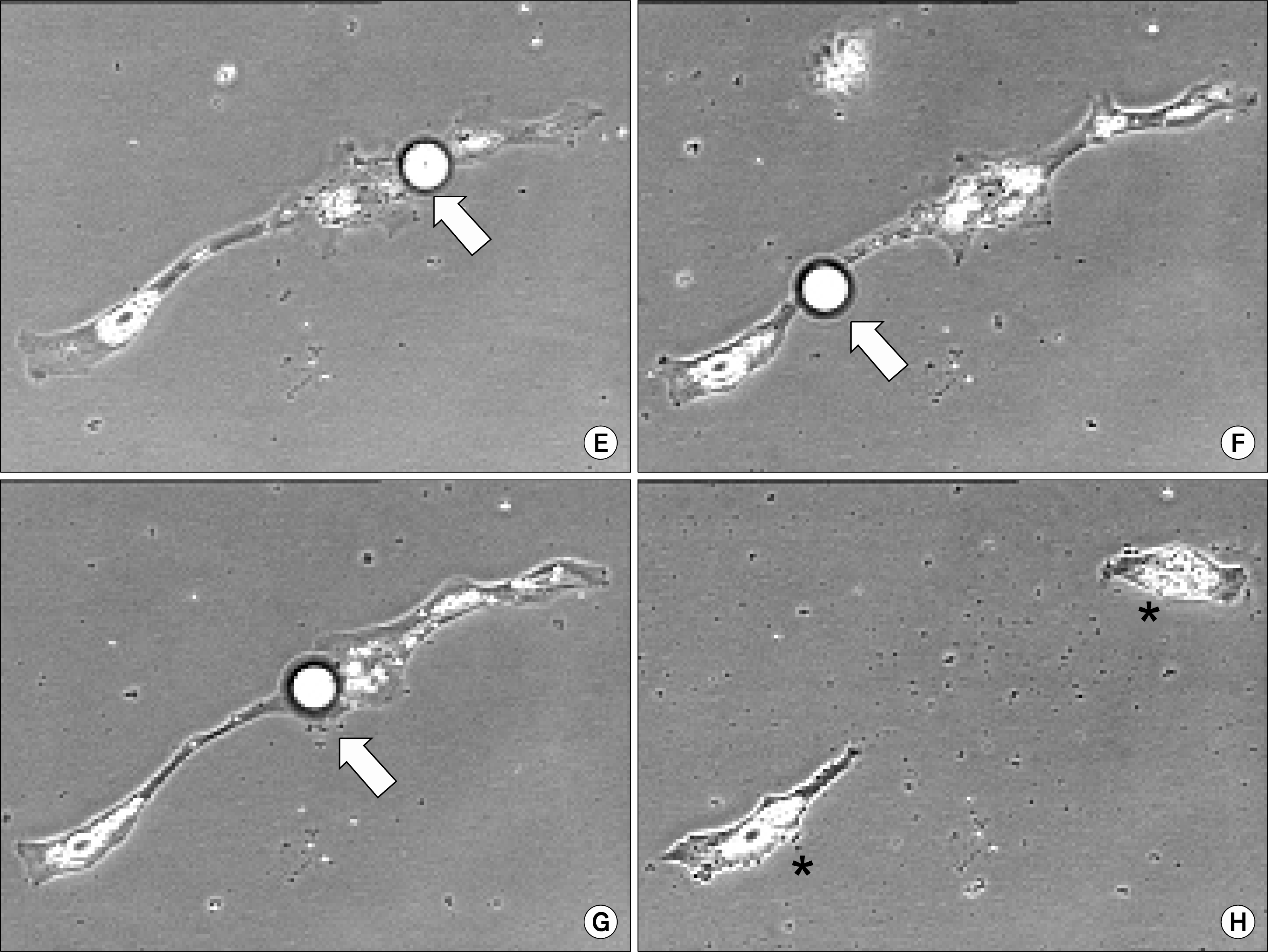
When pigs were used to obtain mature adipocytes for evaluation of this dedifferentiation phenomenon, something quite different was observed (Fig. 1). First, the initial ceiling cultures of pig-derived adipocytes provided qualitatively more mature adipocytes than in the beef system. Second, isolated mature pig-derived adipocytes seemed to include cells with all levels of lipid filling. Third, prior to division, clonal cultures (3) of pig-derived adipocytes were shown to eliminate substantial lipid from the cell, resulting in only an asymmetric cell division. Similar observations were not observed in the beef-derived mature adipocyte cultures (3–5) and suggest that the lipid handling machinery is different and the proliferative machinery of pig-derived adipocytes require less cytoplasmic lipid loads in order to complete the process of cell division.
In part, these types of data offer new insight into species differences in the process of reverse adipogenesis (or de-differentiation), as well as processing of lipid prior to resumption of cell division. This research with clonal populations of proliferative-competent pig-derived adipocytes may prove to be an emerging area of growth physiology that may modify present thinking about adipose tissue renewal capabilities in animals (within and between species). Moreover, it presents a model system for the study of lipid metabolism and mobilization. As adipose tissue provides a reservoir for obtaining putative stem cells (24), knowledge of cellular lipid processing dynamics may aid in our generation of viable stem cells.
References
1. Hausman GJ, Dodson MV, Ajuwon K, Azain M, Barnes KM, Guan LL, Jiang Z, Poulos SP, Sainz RD, Smith S, Spurlock M, Novakofski J, Fernyhough ME, Bergen WG. Board invited review: The biology and regulation of preadipocytes and adipocytes in meat animals. Anim Sci. 2009. 87:1218–1246.

2. Kokta TA, Dodson MV, Gertler A, Hill RA. Intercellular signaling between adipose tissue and muscle tissue. Domest Anim Endocrinol. 2004. 27:303–331.

3. Fernyhough ME, Vierck JL, Hausman GJ, Mir PS, Okine EK, Dodson MV. Primary adipocyte culture: adipocyte purification methods may lead to a new understanding of adipose tissue growth and development. Cytotechnology. 2004. 46:163–172.

4. Fernyhough ME, Helterline DL, Vierck JL, Hausman GJ, Hill RA, Dodson MV. Dedifferentiation of mature adipocytes to form adipofibroblasts: more than just a possibility. Adipocytes. 2005. 1:17–24.
5. Fernyhough ME, Bucci LR, Hausman GJ, Antonio J, Vierck JL, Dodson MV. Gaining a solid grip on adipogenesis. Tissue Cell. 2005. 37:335–338.

6. Dodson MV, Fernyhough ME. Mature adipocytes: are there still novel things that we can learn from them? Tissue Cell. 2008. 40:307–308.

7. Fernyhough ME, Vierck JL, Dodson MV. Assessing a non-traditional view of adipogenesis: adipocyte dedifferentiation-mountains or molehills? Cells Tissues Organs. 2006. 182:226–228.

8. Dodson MV, Fernyhough ME, Vierck JL, Hausman GJ. Adipocytes may not be a terminally differentiated cell type: Implications for animal production. Anim Sci. 2005. 80:239–240.

9. Suryawan A, Hu CY. The primary cell culture system for preadipocytes, in The Biology of Fat in Meat Animals. Smith SB, Smith DR, editors. American Society of Animal Science. Champaign: Illinois;1995. 78–92.
10. Vierck JL, McNamara JP, Dodson MV. Two alternative procedures to isolate adipofibroblasts from sheep skeletal muscle. Method Cell Sci. 1996. 18:309–314.

11. Cancello R, Pietri-Rouxel F, Clement K. Spontaneous lipid accumulation in primary cultures of dedifferentiated human adipocytes. Adipocytes. 2005. 1:73–78.
12. Tholpady SS, Aojanepong C, Llull R, Jeong JH, Mason AC, Futrell JW, Ogle RC, Katz AJ. The cellular plasticity of human adipocytes. Ann Plast Surg. 2005. 54:651–656.

13. Fernyhough ME, Okine E, Hausman G, Vierck JL, Dodson MV. PPARγ and GLUT-4 expression as developmental regulators/markers for preadipocyte differentiation into an adipocyte. Domest Anim Endocrinol. 2007. 33:367–378.

14. Justesen J, Pedersen SB, Stenderup K, Kassem M. Subcutaneous adipocytes can differentiate into bone-forming cells in vitro and in vivo. Tissue Eng. 2004. 10:381–391.

15. Vierck JL, McNamara JP, Dodson MV. Proliferation and differentiation of progeny of ovine unilocular fat cells (adipofibroblasts). In Vitro Cell Dev Biol Anim. 1996. 32:564–572.

16. Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000. 164:119–128.

17. Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986. 31:42–49.

18. Sugihara H, Yonemitsu N, Miyabara S, Toda S. Proliferation of unilocular fat cells in the primary culture. J Lipid Res. 1987. 28:1038–1045.

19. Sugihara H, Yonemitsu N, Toda S, Miyabara S, Funatsumaru S, Matsumoto T. Unilocular fat cells in three-dimensional collagen gel matrix culture. J Lipid Res. 1988. 29:691–697.

20. Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y. A simple culture method of fat cells from mature fat tissue fragments. J Lipid Res. 1989. 30:1987–1995.

21. Adebonojo FO. Studies on human adipose cells in culture: relation of cell size and multiplication to donor age. Yale J Biol Med. 1975. 48:9–16.
23. Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008. 188:359–372.

24. Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008. 368:455–457.

25. Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008. 332:435–446.

26. Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008. 215:210–222.

Fig. 1.
Pig-derived mature adipocyte proliferation in vitro. Basic differences were observed with beef-derived mature adipocytes. The culture method for both the beef and the pig cell systems were as described previously by Fernyhough et al. (3–5). Pig perirenal adipose tissue was enzymatically dispersed, and the lipid-laden fat cells were subjected to ceiling culture and eventually purified using two serial differential plating within 4 d of initial isolation. The purified mature adipocytes were exposed to DMEM/F12+10% FBS medium and monitored daily for morphological changes in the fat cells. Panel (A): the appearance of a bi-locular adipocyte in ceiling culture at 48 h after initating the second ceiling culture. The cell extended and attached firmly to the cultureware. Panel (B), (C), (D): At day 3, 5, 7 (respectively) after initiating the second ceiling culture, the cytoplasm stretched as the cell elongated. A small portion of cellular lipid was observed moving from the central part of the cell to one end (black arrowheads). Panel (E), (F), (G): At day 9, 10, and 11 (respectively) most of the cellular lipid was extruded out of the cell (white arrows). Panel (H): On day 12 after ceiling culture, the cell divided into two daughter cells (asterisks) after expelling a large amount of cellular lipid. All photomicrographs were captured at 20X magnification with a NIKON inverted Diaphot microscope equipped with Sony RGB (0.6 in chip) camera and OPTIX image analysis system.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download