1. Hihi AK, Michalik L, Wahli W. PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci. 2002. 59:790–798.

2. Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. Int J Dev Biol. 2002. 46:105–114.
3. Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990. 347:645–650.

4. Borel V, Gallot D, Marceau G, Sapin V, Blanchon L. Placental implications of peroxisome proliferator-activated receptors in gestation and parturition. PPAR Res. 2008. 758–562.

5. Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal
β-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992. 68:879–887.

6. Akiyama TE, Meinke PT, Berger JP. PPAR ligands: potential therapies for metabolic syndrome. Curr Diab Rep. 2005. 5:45–52.

7. Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004. 1:61–70.

8. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000. 14:1293–1307.

9. Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-
β/
δ in epithelial cell growth and differentiation. Cell Signal. 2006. 18:9–20.

10. Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995. 83:835–839.

11. Bishop-Bailey D, Hla T, Warner TD. Bisphenol a diglycidyl ether (BADGE) is a PPAR
γ agonist in an ECV304 cell line. Br J Pharmacol. 2000. 131:651–654.

12. Buchan KW, Hassall DG. PPAR agonists as direct modulators of the vessel wall in cardiovascular disease. Med Res Rev. 2000. 20:350–366.

13. Huang JC. The role of peroxisome proliferator-activated receptors in the development and physiology of gametes and preimplantation embryos. PPAR Res. 2008. 732303.

14. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000. 405:421–424.

15. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999. 20:649–688.

16. Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of PPAR
δ in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta. 2005. 1740:313–317.

17. Jehl-Pietri C, Bastie C, Gillot I, Luquet S, Grimaldi PA. Peroxisome-proliferator-activated receptor
δ mediates the effects of long-chain fatty acids on post-confluent cell proliferation. Biochem J. 2000. 350:93–98.

18. Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor
δ activates fat metabolism to prevent obesity. Cell. 2003. 113:159–170.

19. Oliver WR Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, Xu HE, Sternbach DD, Kliewer SA, Hansen BC, Willson TM. A selective peroxisome proliferator-activated receptor
δ agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001. 98:5306–5311.

20. Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, Fluhmann B, Desvergne B, Wahli W. Critical roles of PPAR β/δ in keratinocyte response to inflammation. Genes Dev. 2001. 15:3263–3277.
21. Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, Peters JM, Kaya G, Gonzalez FJ, Zakany J, Metzger D, Chambon P, Duboule D, Wahli W. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)
α and PPAR
β mutant mice. J Cell Biol. 2001. 154:799–814.

22. Di-Poi N, Michalik L, Tan NS, Desvergne B, Wahli W. The anti-apoptotic role of PPARβ contributes to efficient skin wound healing. J Steroid Biochem Mol Biol. 2003. 85:257–265.
23. Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-
δ deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004. 10:1245–1250.

24. Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor
δ controls muscle development and oxidative capability. FASEB J. 2003. 17:2299–2301.

25. Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPAR δ. PLos Biol. 2004. 2:e294.
26. Hollingshead HE, Borland MG, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-
β/
δ (PPAR
β/
δ) and inhibition of cyclooxygenase 2 (COX2) attenuate colon carcino-genesis through independent signaling mechanisms. Carcinogenesis. 2008. 29:169–176.

27. Daikoku T, Tranguch S, Chakrabarty A, Wang D, Khabele D, Orsulic S, Morrow JD, Dubois RN, Dey SK. Extracellular signal-regulated kinase is a target of cyclooxygenase-1-peroxisome proliferator-activated receptor-
δ signaling in epithelial ovarian cancer. Cancer Res. 2007. 67:5285–5292.

28. Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK, DuBois RN. Crosstalk between peroxisome proliferator-activated receptor
δ and VEGF stimulates cancer progression. Proc Natl Acad Sci U S A. 2006. 103:19069–19074.

29. Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-
δ accelerates intestinal adenoma growth. Nat Med. 2004. 10:245–247.

30. Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin mediated activation of peroxisome proliferator-activated receptor
δ in colorectal cancer. Proc Natl Acad Sci U S A. 2000. 97:13275–13280.

31. Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPAR
δ. Science. 2003. 302:453–457.

32. Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPAR
α,
β/
δ, and
γ. J Clin Invest. 2004. 114:1564–1576.

33. Liou JY, Lee S, Ghelani D, Matijevic-Aleksic N, Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-
δ mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006. 26:1481–1487.

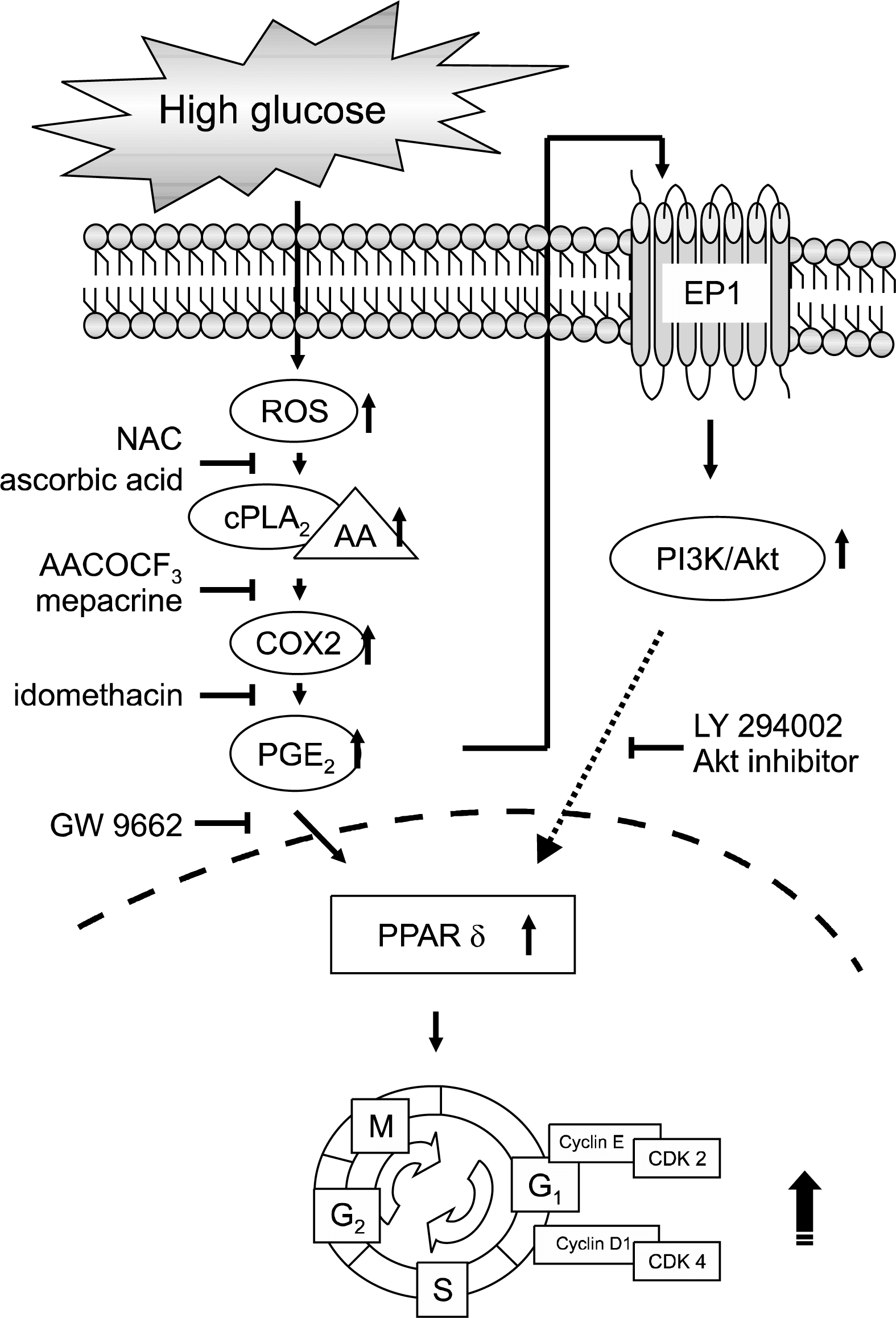
34. Kim YH, Han HJ. High-glucose-induced prostaglandin E
2 and peroxisome proliferator-activated receptor
δ promote mouse embryonic stem cell proliferation. Stem Cells. 2008. 26:745–755.

35. Glazer RI, Yuan H, Xie Z, Yin Y. PPARγ and PPARδ as modulators of neoplasia and cell fate. PPAR Res. 2008. 247379.
36. Huang JC, Wun WS, Goldsby JS, Wun IC, Noorhasan D, Wu KK. Stimulation of embryo hatching and implantation by prostacyclin and peroxisome proliferator-activated receptor
δ activation: implication in IVF. Hum Reprod. 2007. 22:807–814.

37. Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPAR
δ. Genes Dev. 1999. 13:1561–1574.

38. Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-
α, -
β, and -
γ during rat embryonic development. Endocrinol. 1998. 139:2748–2754.

39. Higa R, González E, Pustovrh MC, White V, apobianco E, Martínez N, Jawerbaum A. PPAR
δ and its activator PGI2 are reduced in diabetic embryopathy: involvement of PPAR
δ activation in lipid metabolic and signalling pathways in rat embryo early organogenesis. Mol Hum Reprod. 2007. 13:103–110.

40. Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-
α,-
β, and -
γ in the adult rat. Endocrinol. 1996. 137:354–366.

41. Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci U S A. 1994. 91:7355–7359.

42. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors
α and
δ. Proc Natl Acad Sci U S A. 1997. 94:4312–4317.

43. Lee CH, Kang K, Mehl IR, Nofsinger R, Alaynick WA, Chong LW, Rosenfeld JM, Evans RM. Peroxisome proliferator-activated receptor
δ promotes very low-density lipoprotein-derived fatty acid catabolism in the macrophage. Proc Natl Acad Sci U S A. 2006. 103:2434–2439.

44. Ghosh M, Wang H, Ai Y, Romeo E, Luyendyk JP, Peters JM, Mackman N, Dey SK, Hla T. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPAR
δ activation. J Exp Med. 2007. 204:2053–2061.

45. Shaw N, Elholm M, Noy N. Retinoic acid is a high affinity selective ligand for the peroxisome proliferator-activated receptor
β/
δ. J Biol Chem. 2003. 278:41589–41592.

46. Basséne CE, Suzenet F, Hennuyer N, Staels B, Caignard DH, Dacquet C, Renard P, Guillaumet G. Studies towards the conception of new selective PPARβ/δ ligands. Bioorg Med Chem Lett. 2006. 16:4528–4532.
47. Bishop-Bailey D, Wray J. Peroxisome proliferator-activated receptors: a critical review on endogenous pathways for ligand generation. Prostaglandins Other Lipid Mediat. 2003. 71:1–22.

48. Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006. 58:726–741.

49. Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, Sierra ML, LeGrumelec C, Xu HE, Montana VG, Lambert MH, Willson TM, Oliver WR Jr, Sternbach DD. Novel selective small molecule agonists for peroxisome proliferator-activated receptor
δ (PPAR
δ)-synthesis and biological activity. Bioorg Med Chem Lett. 2003. 13:1517–1521.

50. Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, Boland R, Evans RM. Effects of peroxisome proliferator-activated receptor
δ on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci U S A. 2002. 99:303–308.

51. Peters JM, Lee SS, Li W, Ward JM, Gavrilova O, Everett C, Reitman ML, Hudson LD, Gonzalez FJ. Growth, adi-pose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor
β (
δ). Mol Cell Biol. 2000. 20:5119–5128.

52. Kopelovich L, Fay JR, Glazer RI, Crowell JA. Peroxisome proliferator-activated receptor modulators as potential chemopreventive agents. Mol Cancer Ther. 2001. 1:357–363.
53. He TC, Chan TA, Vogelstein B, Kinzler KW. PPAR
δ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999. 99:335–345.

54. Zhang J, Fu M, Zhu X, Xiao Y, Mou Y, Zheng H, Akinbami MA, Wang Q, Chen YE. Peroxisome proliferator-activated receptor
δ is up-regulated during vascular lesion formation and promotes post-confluent cell proliferation in vascular smooth muscle cells. J Biol Chem. 2002. 277:11505–11512.

55. Hansen JB, Zhang H, Rasmussen TH, Petersen RK, Flindt EN, Kristiansen K. Peroxisome proliferator-activated receptor
δ (PPAR
δ)-mediated regulation of preadipocyte proliferation and gene expression is dependent on cAMP signaling. J Biol Chem. 2001. 276:3175–3182.

56. Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-
δ and prostaglandin E
2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006. 281:33982–33996.

57. Burdick AD, Bility MT, Girroir EE, Billin AN, Willson TM, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-
β/
δ (PPAR
β/
δ) inhibits cell growth of human N/TERT-1 keratinocytes. Cell Signal. 2007. 19:1163–1171.

58. Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPAR
δ activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003. 308:361–368.

59. Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, Das SK, Dey SK, DuBois RN. Prostaglandin E
2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor
δ. Cancer Cell. 2004. 6:285–295.

60. Wang H, Xie H, Sun X, Tranguch S, Zhang H, Jia X, Wang D, Das SK, Desvergne B, Wahli W, DuBois RN, Dey SK. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor
δ signaling is critical to pregnancy success. J Biol chem. 2007. 282:37770–37782.

61. Pakrasi PL, Jain AK. Evaluation of cyclooxygenase 2 derived endogenous prostacyclin in mouse preimplantation embryo development in vitro. Life Sci. 2007. 80:503–1507.

62. Cimini A, Benedetti E, Cristiano L, Sebastiani P, D'Amico MA, D'Angelo B, Di Loreto S. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005. 30:325–337.

63. Shi Y, Hon M, Evans RM. The peroxisome proliferators-activated receptor
δ an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci U S A. 2002. 99:2613–2618.

64. Cimini A, Cristiano L, Benedetti E, D'Angelo B, Cerú MP. PPARs expression in adult mouse neural stem cells: modulation of PPARs during astroglial differentiaton of NSC. PPAR Res. 2007. 48242.

65. IJpenberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, Metzger D, Canaple L, Chambon P, Wahli W, Desvergne B. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004. 23:2083–2091.

66. Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006. 45:120–159.

67. Wang XY, Yin Y, Yuan H, Sakamaki T, Okano H, Glazer RI. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol. 2008. 28:3589–3599.

68. Varnat F, Heggeler B, Grisel P, Boucard N, Corthésy-Theulaz I, Wahli W, Desvergne B. PPAR
β/
δ regulates paneth cell differentiation via controlling the hedgehog signaling pathway. Gastroenterology. 2006. 131:538–553.

69. Pakrasi PL, Jain AK. Cyclooxygenase-2 derived PGE
2 and PGI
2 play an important role via EP
2 and PPAR
δ receptors in early steps of oil induced decidualization in mice. Placenta. 2008. 29:523–530.

70. Jawerbaum A, Gonzalez ET, Sinner D, Pustovrh D, White V, Gimeno MA. Diminished PGE
2 content, enhanced PGE
2 release and defects in
3H-PGE
2 transport in embryos from overtly diabetic rats. Reprod Fertil Dev. 2000. 12:141–147.

71. Kennedy TG. Evidence for the involvement of prostaglandins throughout decidual cell reaction in the rat. Biol Reprod. 1985. 33:140–146.

72. Tawfik OW, Sagritlo C, Johnson DC, Dey SK. Decidualization in the rat: role of leukotrienes and prostaglandins. Prostaglandins Leukot Med. 1987. 29:221–227.
73. Papay KD, Kennedy TG. Characterizatio of temporal and cell specific changes in transcripts for prostaglandin E(2) receptor in pseudopregnant rat endometrium. Biol Rprod. 2000. 62:1515–1525.

74. Burdon T, Smith A, Savatier P. Signaling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002. 12:432–438.
75. Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveirados-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3KPTEN signaling pathways. Cell. 2002. 110:737–749.

76. Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999. 18:4261–4269.

77. Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999. 96:2846–2851.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download