Introduction
Materials and Methods
Cell culture
Induction of nestin-expressing spheroids
Immunocytochemical staining
Statistical analysis
Results
Efficient induction of nestin-expressing spheroids from HDFs using a two-step induction method
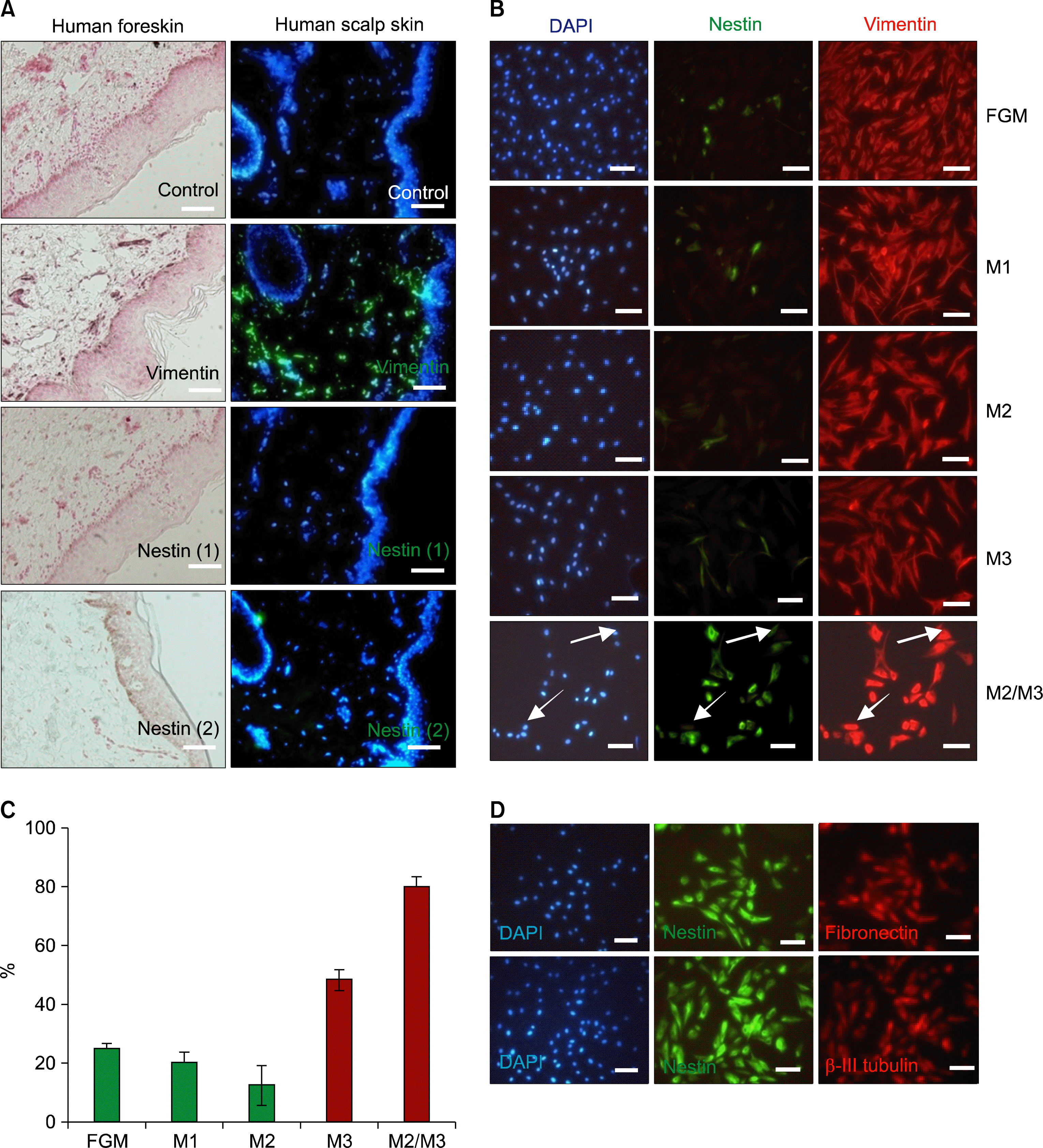 | Fig. 1.Induction of nestin, FN and β-III tubulin in HDFs by a novel two-step process. (A) Endogenous expression of nestin and vimentin in a formalin-fixed paraffin section of human newborn foreskin and a frozen section of human scalp tissue. Immunohistochemical staining for vimentin and nestin in paraffin sections of human foreskin developed by DAB and counterstained with Fast Red. Immunofluorescence staining against vimentin and nestin in a frozen section of human scalp was detected with FITC-conjugated secondary antibodies and counter-stained with DAPI. Two different nestin (nestin (1), Chemicon; nestin (2), Biodesign) antibodies were used. Brown-staining in the basal layer of the epidermis was due to melanin deposition, as shown in the control sample. (B) Expression of nestin and vimentin in HDFs derived from newborn foreskin. The cells were cultured under different conditions as described in materials and method. After acetone/ethanol fixation, double immunofluorescence staining with anti-nestin and anti-vimentin were performed, followed by DAPI counterstaining. Nestin-negative but vimentin-positive cells are noted by arrows. (C) Statistical comparison of total nestin-expressing versus DAPI-positive cells. Results are expressed as means±S.D, for three independent experiments. Cells cultured in M2/M3 showed significantly higher rates of nestin expression compared to other groups (P<0.05). (D) Double immunofluorescence staining of nestin-expressing cells obtained via two-step induction, showing expression of fibronectin and β-III tubulin. The cells were cultured under two-step induction condition and double immunofluorescence staining with anti-nestin and anti-fibronectin antibodies or anti-nestin and anti β-III tubulin were performed. Scale bar A 400 μm, B and D 100 μm. |
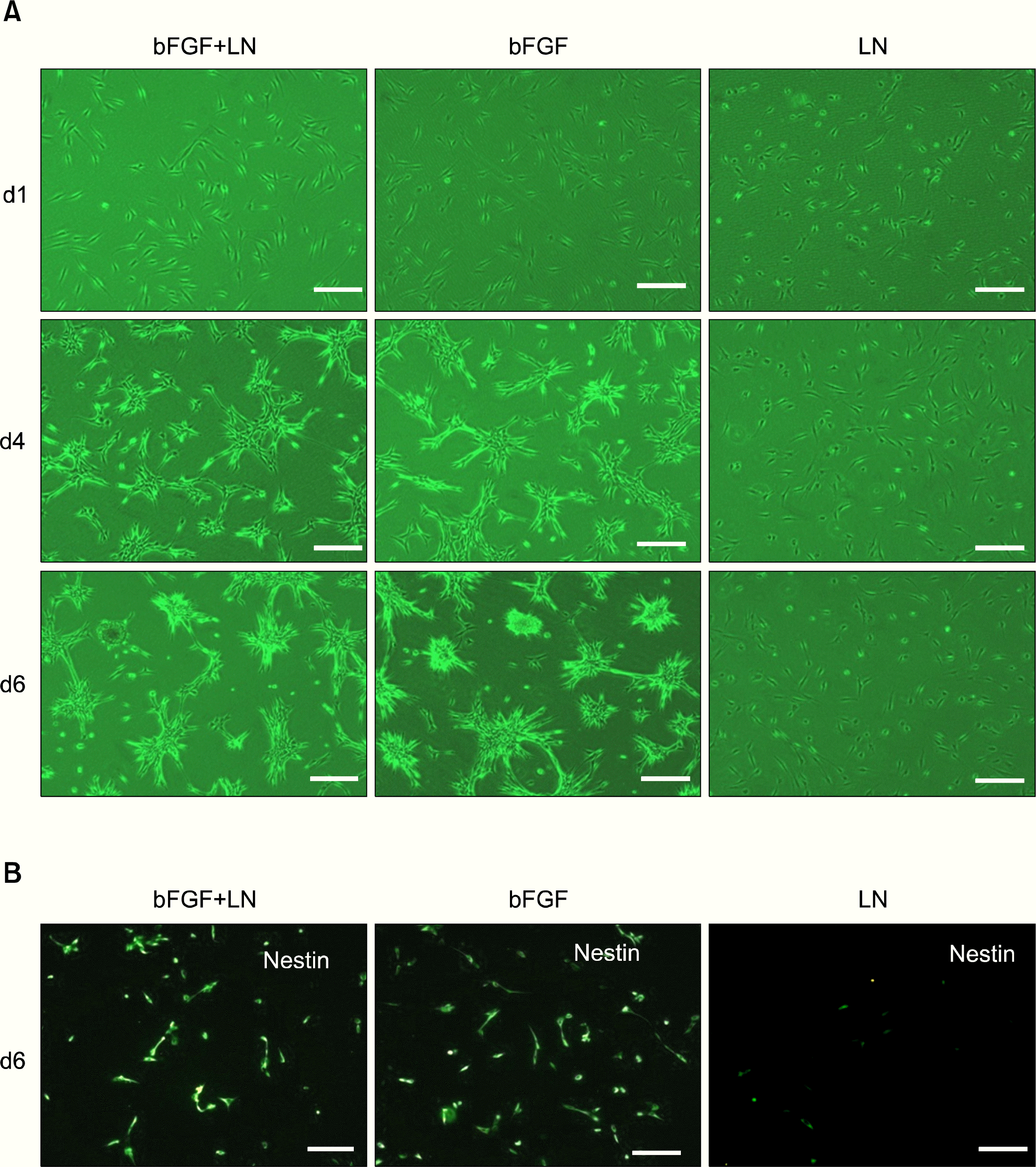
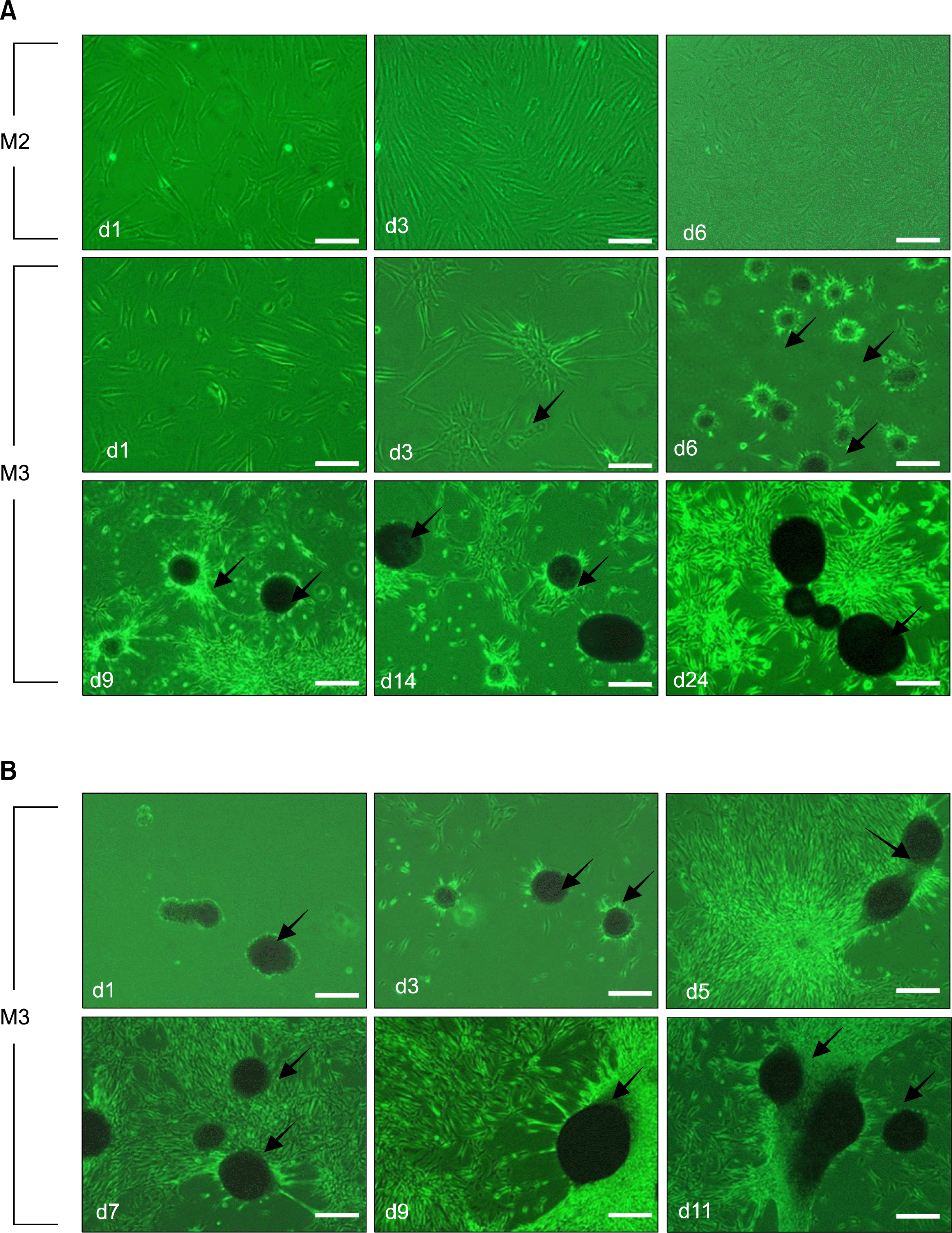 | Fig. 2.Specific induction of spheroids during incubation in M3 medium containing bFGF and laminin (LN), after priming in serum-free M2 medium. (A) Comparison of spheroid-forming capacities in M2 versus M3 media. HDFs did not form spheroids in M2 medium, but were induced to form spheroids within six days after transfer to M3 medium. Centripetal upward attraction of the cell sheet (arrow), which is an early sign of spheroid formation, is noted by an arrow. Obvious spheroid (arrows) formation was discernible at 6 days. (B) Long-term maintenance of spheroid-forming capacity. Spheroids were split into small clumps or single cells that reformed as spheroids(arrows) for up to eight weeks. Scale bar A 400 μm. |
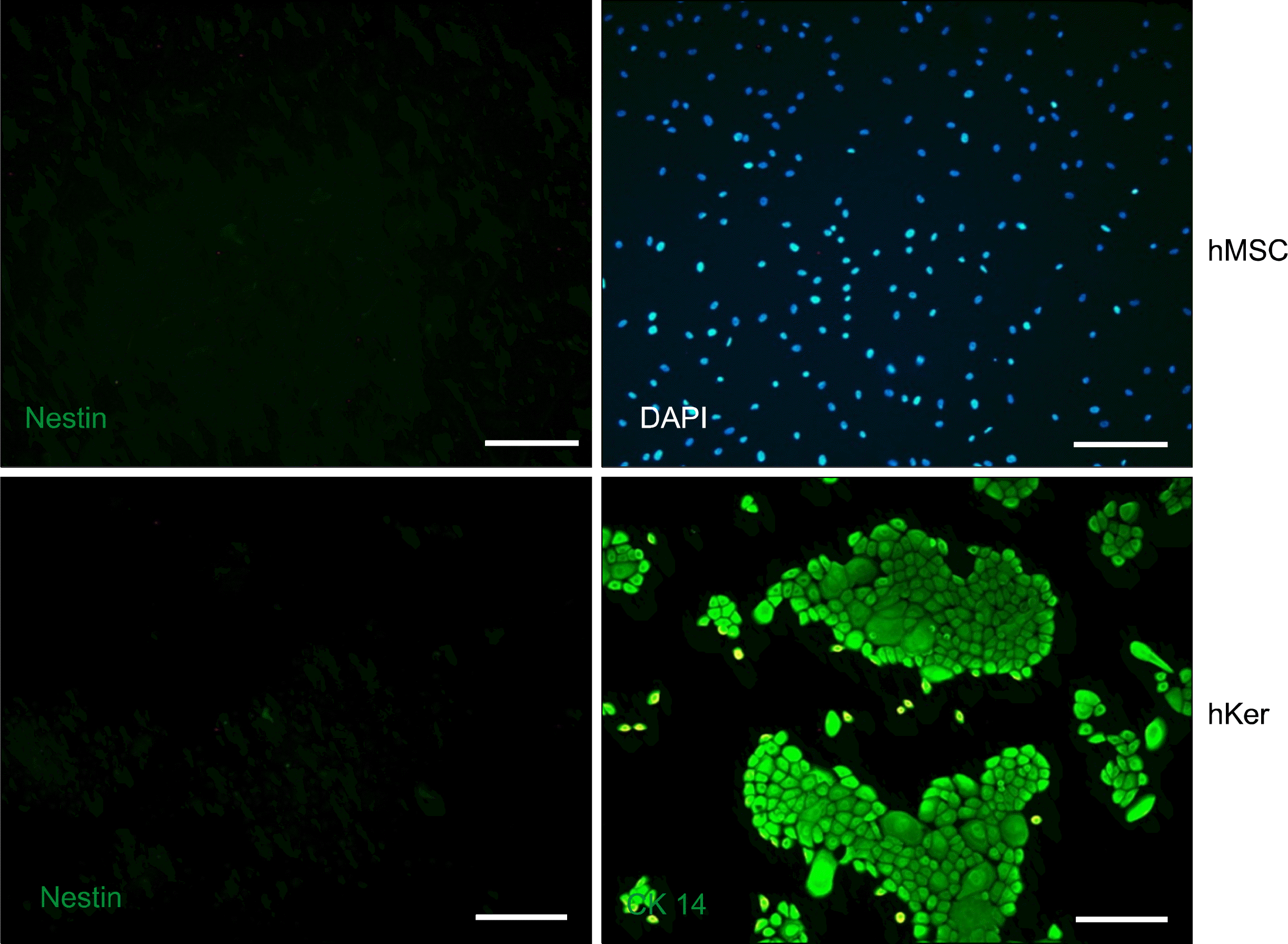
 | Fig. 3.Characterization of nestin-expressing and spheroid-forming cells after long term culture. (A) Immunocytochemical staining of nestin-expressing spheroid-forming cells with anti-nestin, anti-vimentin, anti- β-III tubulin, anti-A2B5, anti-p75, anti-O4, anti-MAP2, anti-Tau, anti-NF-150, anti-NF-68, and anti-GFAP antibodies after 40 day spheroid culture. (B) Immunohistochemical staining of spheroids with anti-A2B5, anti-p75, anti-O4, anti-NF-68, and anti-GFAP antibodies. Scale bar A 100 μm, B and D 200 μm. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download