Abstract
The greatest advantage of adipose-derived stem cells (ASCs) over other types of adult stem cells is its large number when we harvest primarily. The number of ASCs within adipose tissue reaches more than hundreds of times compared with BMSCs contained in the same amount of bone marrow. The major role of ‘regenerative medicine’ in 21st century is based on cell therapy and ASC is going to take the core position. It is important to know the characteristics of ASCs for successful clinical application. There are several unique features of ASCs which is known common characteristic of mesenchymal stem cells (MSCs). Cellular plasticity is one of the most important features of ASCs as in other adult stem cells and the cells also have a special function of immune modulation and immunosuppression. Strong angiogenic potential is another important nature of ASCs. In many reports, ASCs are known not only to be differentiated into osteoblasts, chondrocytes, vascular endothelial cells, but also to be cardiomyocytes and neuronal cells. In conclusion, the new knowledge of ASCs is going to impact on the regenerative medicine. To take the advantage of this new type of cells and utilize the cells, we need to understand the function of ASCs and future possibilities of ASCs. We plastic surgeons continue to stimulate the our curiosity and creativity, as well as our clinical inspiration.
There had been an important scientific discovery of adipose stem cell (ASCs) at Pittsburgh University during 1997∼1998. Several curious Plastic surgeons of Pittsburgh University had found very interesting type of stromal cells in large numbers while they were working on cells harvested from human lipoaspirate. The stromal cells could be differentiated into osteogenic lineages in osteogenic culture media. The cells also could be differentiated into myogenic lineages in myogenic media containing horse serum. They started to investigate this interesting type of multi-potent stromal cells and it was the beginning of historical discovery of ASCs.
During the last several decades, bone marrow stem cells (BMSCs) have been studied by many outstanding scientists and the results were quite promising. However, the amount of BMSCs were so few scientist had problem in proliferating the cells to therapeutic number. As the number of passage increases to 5∼6, changes in cellular characteristics occurred. So, scientists have been struggling to proliferate BMSCs without deteriorating the nature of cells and actual clinical application had been markedly delayed. At this critical period, the discovery of ASCs occurred and the cells share common characteristics as adult stem cell and they could be easily harvested in great numbers from human adipose tissue. Now, clinical application of stem cell therapy is almost within our reach.
All sample collection procedures from human beings and experimental protocols were approved by the institute. Before tissue digestion, harvested fat tissue was briefly washed with equal volumes of phosphate-buffered saline (PBS). Enzymatic tissue digestion was done with 0.075% collagenase type I (Worthington Biochemical, USA) at 37°C for 30 min. Digested tissue was filtered to remove connective tissue debris. After separation of floating mature adipose cell layer, cell suspension was centrifuged at 200 g force for 10 minutes and the cell pellet was recovered and washed. The contaminated red blood cells were eliminated by adding an erythrocyte lysis buffer pH 7.3. The stromal cells were rinsed twice with PBS and seeded at a density of 2 to 3×105/cm2 onto a culture flask in a complete medium consisting of low glucose DMEM (Dulbecco’s Modified Eagle Medium, Gibco-BRL, USA) with 10% fetal bovine serum (FBS) and 100 U/ml penicillin and streptomycin
Cultures were kept at 37°C with humid 5% CO2 air atmosphere chamber. After 2∼3 days of initial seeding, hASCs adhered to the plastic surface, presenting a small population of single cells. The adherent single ASCs demonstrated a spindle-shape or fibroblast-like morphology with one nucleus (Fig. 1). The first media change was done after 24 hours to remove unattached cells and then the attached stem cells were fed every three days. Six days after initial plating the cells looked like long spindle-shaped fibroblastic cells and began to form colonies. As the number of the cells increase they becomes confluent (Fig. 2) and passage is necessary for further increase in number.
In the flow cytometric analysis, hASCs were consistently positive for the following adhesion molecules: CD13, CD29, CD44 and CD90, CD166 and HLA-ABC (Fig. 3) which together is considered as marker for mesenchymal stem cells. In contrast, the cells were negative for hematopoietic lineage markers such as CD31, CD34, CD45, CD117 and HLA-DR. (Fig. 4). Result of flow cytometric analysis is shown in Table 1.
According to my own experiments and many other reports on literature, ASCs have similar cellular plasticity of other adult stem cells such as bone marrow stem cells. Many pioneering researchers have demonstrated that adipose tissue contains a large number of adult stem cells capable of differentiation into multiple lineages (1), including adipocytes, chondrocytes (2, 3), osteoblasts (4, 5), endothelial cells (6), and hepatocytes (7). Cardiomyogenic differentiation was described by Rangappa et al. and they have utilized a kind of cytosine analogue, 5-azacytidine, which demethylizing DNA and transform a certain control gene to initiate differentiation of ASCs into cardiomyocytes (8). In recent studies, the cells are capable of regenerate not only many other mesenchymal tissues but also neuro-ectodermal and endodermal tissue.
As reported in many literatures, adult stem cells have special characteristic of immuno-modulatory/immuno suppression. The adult stem cells lack HLA-DR which is an important antigenicity of MHC (major histocompatibility complex) and allogeneic or xenogeneic transplantation does not provoke severe immunologic rejection. Actually, in our animal experiment using human ASCs without any immunosuppressive treatment, careful histological examination did not disclose any area of severe inflammation which may be related with immunologic reaction. This unique characteristic of ASCs seems to be very important and the immuno-modulatory/immuno-suppressive function of ASCs opened a new horizon in this field of cell therapy by providing a reliable amount of stem cells as allogeneic cell therapeutic agent.
The stromal cell population harvested from human lipoaspirate is composed of many different types of cells, such as ASCs, vascular endothelial progenitor cells, pre-adipocytes, and other hematologic cells. Among them, ASCs and vascular endothelial progenitor cells are predominant and they are highly angiogenic. The cells rapidly form micro-tubular structure within 24 hours on Matrigel-coated culture dish (Fig. 5). The cells injected into soft tissue with fibrin glue regenerate capillary network within fibrin glue as early as in two weeks (Fig. 6). These capabilities of tissue regeneration and angiogenesis of adult stem cells could be utilized for therapeutic purposes especially for several diseases related with local ischemia-tissue damage such as ischemic heart disease. Ischemic heart disease is a well-known prevailing cause of death in developed countries and the treatment of this disease using the ASCs might be one of the most challenging goals in this field of modern science.
ASCs are capable of regenerate not only many other mesenchymal tissues but also neuro-ectodermal and endodermal tissue. Reports of animal study using cerebral infarction rat model, human ASCs treatment revealed increased motor function recovery. Some scientists are also working on the possibility of peripheral nerve regeneration for better healing of direct nerve repair and nerve gap. If human ASCs could be utilized for motor recovery of brain stroke patients the clinical implication would be magnificent.
In recent study of my own, ASCs have been treated in animal model with acute and chronic renal failure. We concluded ASCs have critical function on the recovery of damaged renal function. In acute renal failure model, it seems ASCs affects recovery of renal function through vasculogenesis. In chronic renal failure model, functional improvement of renal glomerulus was obvious and it seems ASCs induce tubulo-interstitial regeneration.
It is also reported ASCs induce hematopoiesis in rats. After lethal-dose of whole body irradiation, significant numbers of rats survived with intraperitoneal injection of ASCs as in rats with bone marrow transplantation. In the future, leukemia patient may be treated with simply treated with ASCs instead of complicated bone marrow transplantation.
Many experienced Plastic surgeons already realized unexpected effect of facial skin rejuvenation after fat injection on face. The rejuvenating effect is so delicate and obvious it could not be simply explained with volume replacement effect of injected fat. Now, Plastic surgeons explain the effect is due to free mobilized ASCs in fat injection procedure (9). Many cytokines released from damaged fat cells may be a related factor.
ASCs could be utilized as a wound healing promoter. Different from applying wound healing cytokines, application of ASCs within difficult wound directly produce capillaries and new vessels as well as releasing HGF (hypoxia-induced growth factor), VEGF and PDGF. This powerful wound healing mechanism could provide a new solution for radiation dermatitis or radiation necrosis.
Liposuction is one of the most popular cosmetic surgeries in the world and everyday in operating theater, incredible amount of fat tissue is discarded as bio-hazard waste. Now, we all know ASCs are so precious agent worthy banking for future therapeutic purposes. As the cells renew, theoretically, the number of adult stem cells within soft tissue does not decreased. However recent papers report decreased number of adult stem cells within soft tissue for unknown reason. So, it may be necessary to bank reasonable amount of ASCs when we are young. There are already several venture business companies working on banking of ASCs.
According to the discovery of adipose-derived stem cells, it became possible to acquire enough numbers of stem cells as a clinically applicable therapeutic agent even without a culture expansion process. The characteristics of the ASCs are not fully disclosed yet and it seems they have similar cellular plasticity as other types of mesenchymal stem cells. ASCs are already being utilized in Plastic surgery clinics as primary cells and many other medical specialists are testing the cells for clinical purposes such as treatment of ischemic heart disease, recovery of brain stroke and rehabilitation of spine injury. Cell therapy using adult stem cells is becoming one of the ‘core’ elements of the emerging field of regenerative medicine and adipose stem cell is becoming one of the most popular sources of stem cell therapy.
ACKNOWLEDGMENTS
I’d like to express my hearty appreciation to my research assistants, Seil Park, Young-Mi Moon and San-Ok Kim for their devoted services in the laboratory of the department of Plastic and Reconstructive Surgery, Yeungnam University Medical Center, Daegu.
References
1. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001. 7:211–227.

2. Jeong JH. Chondrogenic differentiation of human adipo-derived precursor cells. J Korean Soc Plast Reconstr Surg. 2000. 27:136–142.
3. Erickson GR, Gimble JM, Franklin DM, Rice HE, Awad H, Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun. 2002. 290:763–769.

4. Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extra-cellular matrix mineralization and osteoblasts gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001. 7:729–741.

5. Hicok KC, Du Laney TV, Zhou YS, Halvorsen YD, Hitt DC, Cooper LF, Gimble JM. Human adipose-derived adult stem cells produce osteoid in vivo. Tissue Eng. 2004. 10:371–380.
6. Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004. 109:656–663.

7. Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005. 328:258–264.

Fig. 1.
Microscopic appearance of primary human adipose stem cells (ASCs). The adherent single human ASCs demonstrated a spindle-shape or fibroblast-like morphology with one nucleus (×200).
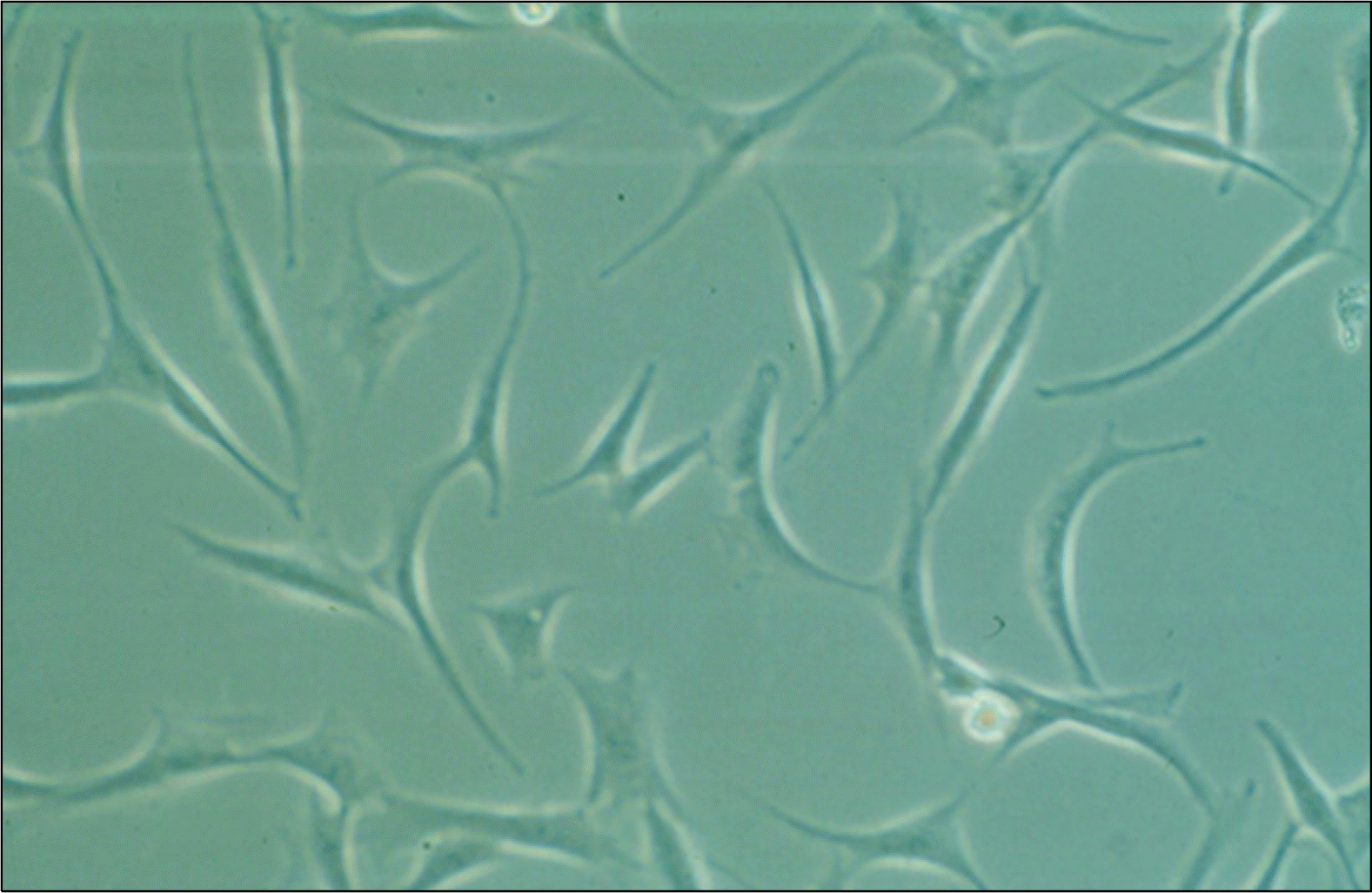
Fig. 2.
Microscopic appearance of cultured primary human adipose stem cells (ASCs) in confluent state. For further culture expansion, passage is necessary (×200).
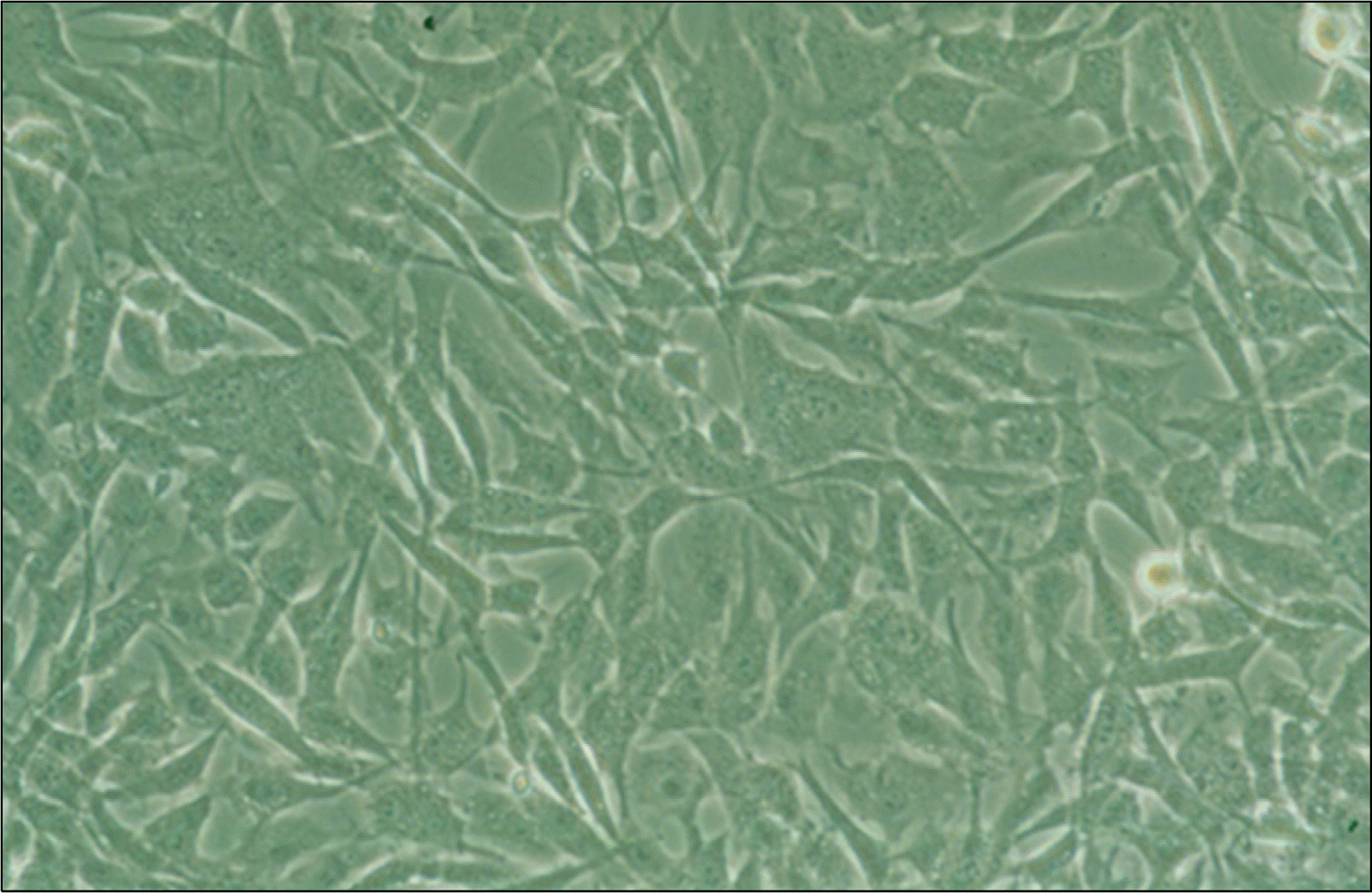
Fig. 3.
Flow cytometric analysis of harvested human adipose-derived stem cells. Cells were stained with monoclonal antibodies directed against CD13, CD29, CD45, CD90 and CD166. HLA-DR and HLA-ABC served as a control for PE.
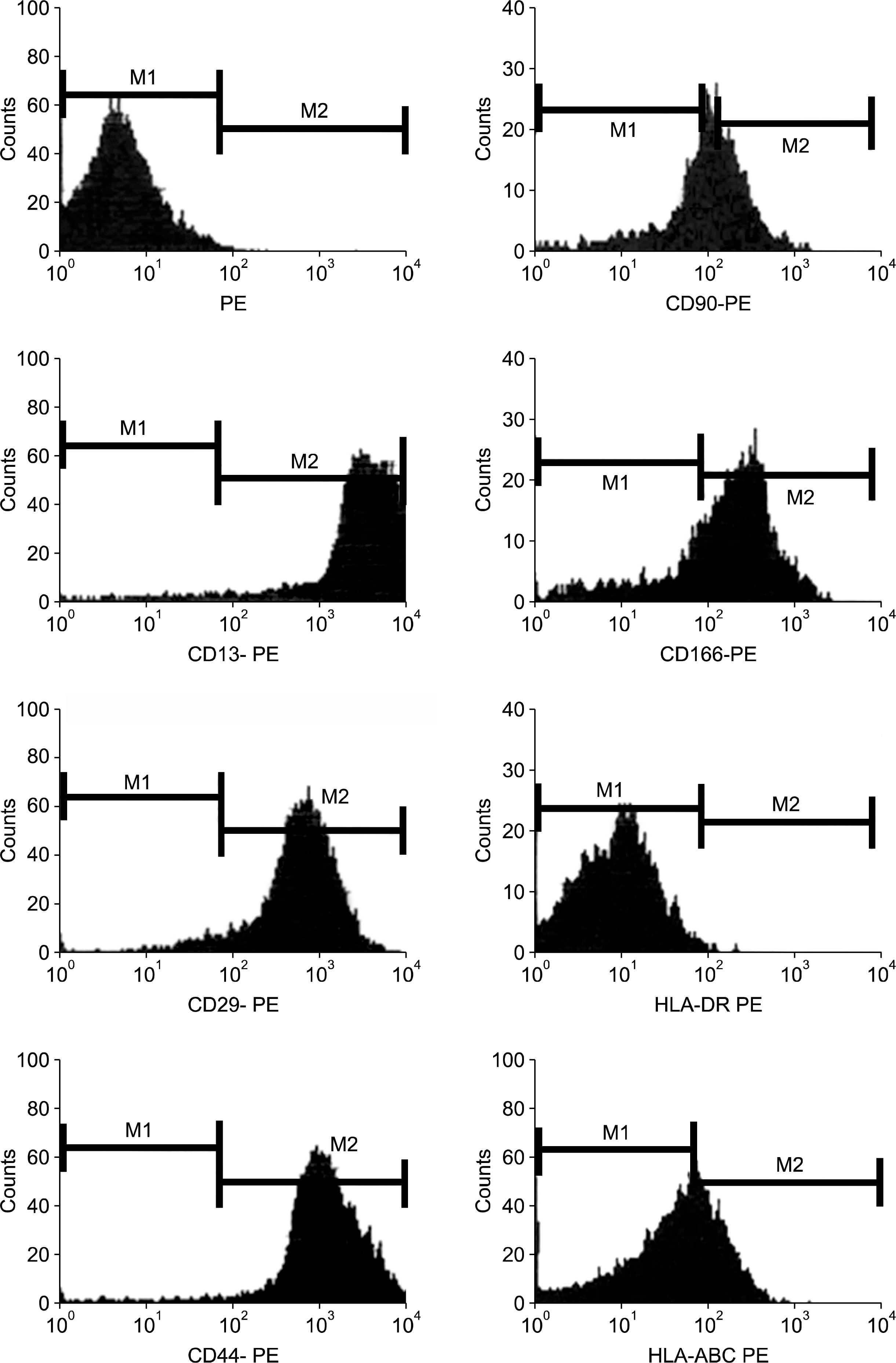
Fig. 4.
Flow cytometric analysis of human adipose derived stem cells. Cells were stained with monoclonal antibodies directed against CD31, CD34, CD45 and CD117 which are known as hematopoietic cell markers.
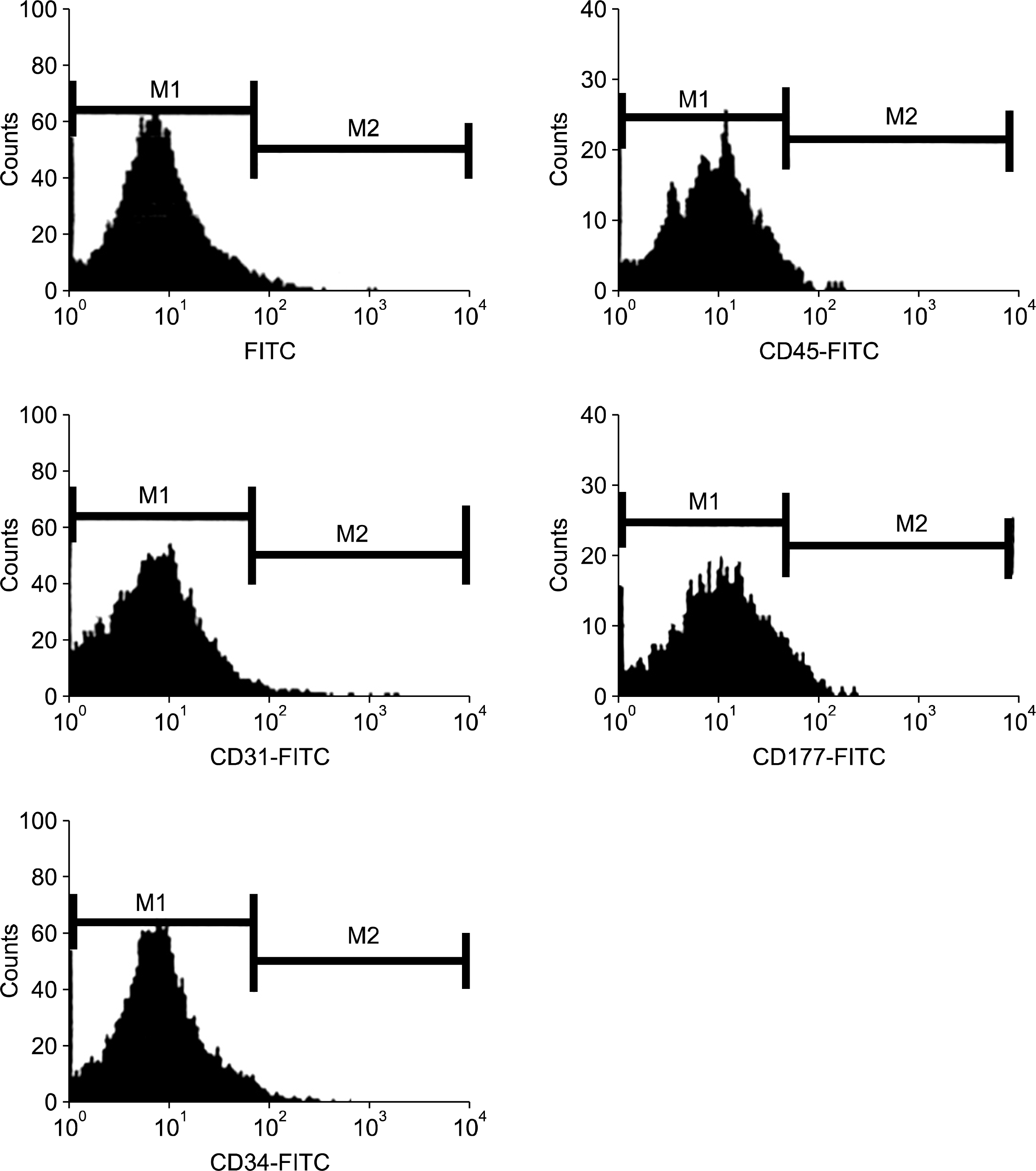
Fig. 5.
Adipose stem cells rapidly transform into micro-tubular structures within 24 hours on Matrigel-coated culture dish.
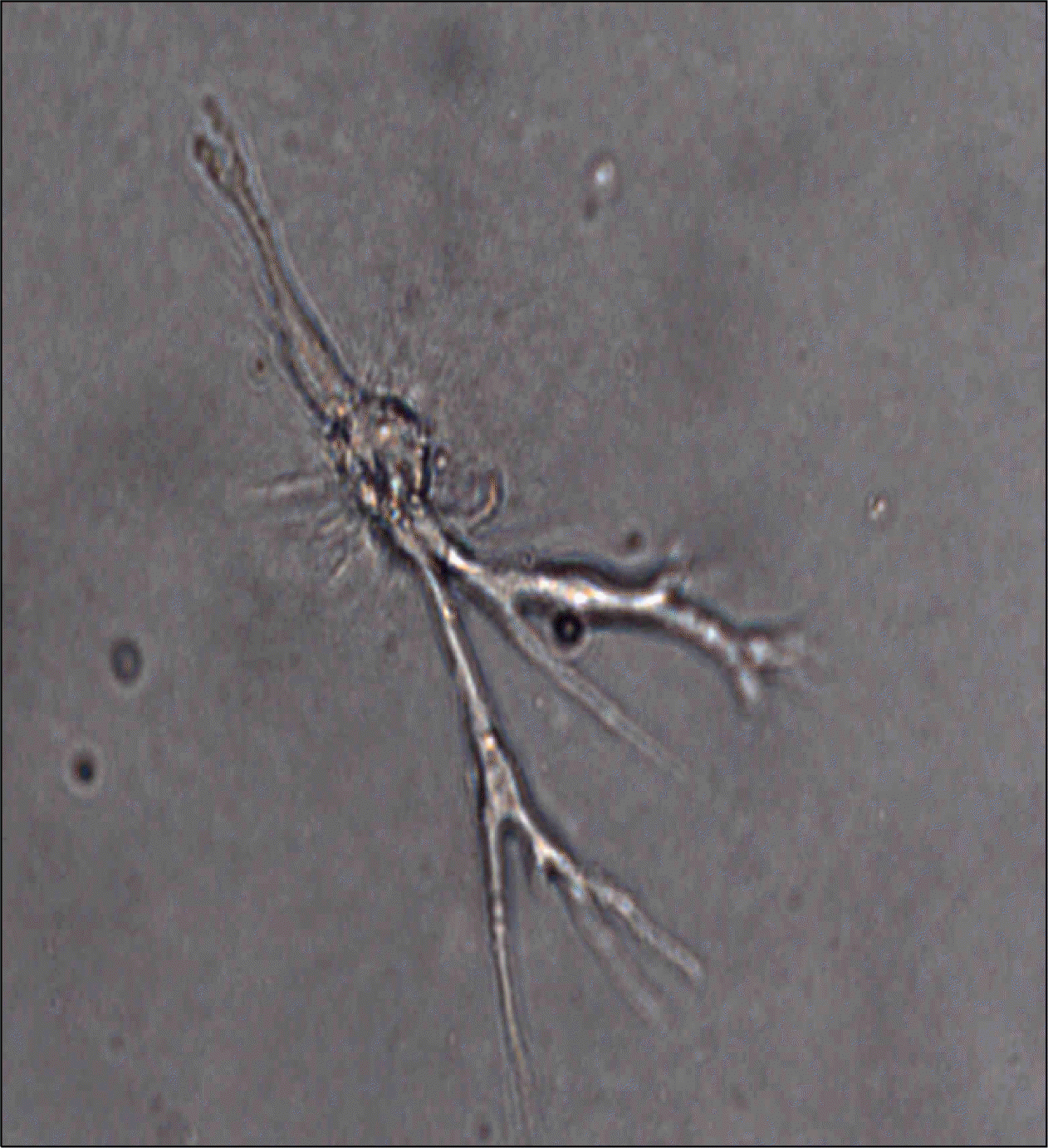
Fig. 6.
Adipose stem cells injected into soft tissue with fibrin glue regenerate capillary network within fibrin glue as early as in two weeks.
Table 1.
Result of flow cytometric analysis of harvested human adipose-derived stem cells described as percentage of positive fluorescence




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download