2. Fernando LP, Kandel PK, Yu J, McNeill J, Ackroyd PC, Christensen KA. 2010; Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles. Biomacromolecules. 11:2675–2682. DOI:
10.1021/bm1007103. PMID:
20863132. PMCID:
PMC2962534.

3. Lee SY, Lee S, Lee J, Yhee JY, Yoon HI, Park SJ, Koo H, Moon SH, Lee H, Cho YW, Kang SW, Lee SY, Kim K. 2016; Non-invasive stem cell tracking in hindlimb ischemia animal model using bio-orthogonal copper-free click chemistry. Biochem Biophys Res Commun. 479:779–786. DOI:
10.1016/j.bbrc.2016.09.132. PMID:
27693784.

4. Han SS, Lee DE, Shim HE, Lee S, Jung T, Oh JH, Lee HA, Moon SH, Jeon J, Yoon S, Kim K, Kang SW. 2017; Physiological effects of Ac4ManNAz and optimization of metabolic labeling for cell tracking. Theranostics. 7:1164–1176. DOI:
10.7150/thno.17711. PMID:
28435456. PMCID:
PMC5399584.

5. Han SS, Shim HE, Park SJ, Kim BC, Lee DE, Chung HM, Moon SH, Kang SW. 2018; Safety and optimization of metabolic labeling of endothelial progenitor cells for tracking. Sci Rep. 8:13212. DOI:
10.1038/s41598-018-31594-0. PMID:
30181604. PMCID:
PMC6123424.

6. Kang SW, Lee S, Na JH, Yoon HI, Lee DE, Koo H, Cho YW, Kim SH, Jeong SY, Kwon IC, Choi K, Kim K. 2014; Cell labeling and tracking method without distorted signals by phagocytosis of macrophages. Theranostics. 4:420–431. DOI:
10.7150/thno.7265. PMID:
24578725. PMCID:
PMC3936294.

8. Karabanovas V, Zitkus Z, Kuciauskas D, Rotomskis R, Valius M. 2014; Surface properties of quantum dots define their cellular endocytic routes, mitogenic stimulation and suppression of cell migration. J Biomed Nanotechnol. 10:775–786. DOI:
10.1166/jbn.2014.1770. PMID:
24734530.

9. Ni SD, Yin YW, Li XL, Ding HM, Ma YQ. 2019; Controlling the interaction of nanoparticles with cell membranes by the polymeric tether. Langmuir. 35:12851–12857. DOI:
10.1021/acs.langmuir.9b02010. PMID:
31474103.

10. Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Müller E, Küest SM, Cohrs S, Schibli R, Kronen P, Hilbe M, Reinisch A, Strunk D, Haverich A, Hoerstrup S, Lüscher TF, Kaufmann PA, Landmesser U, Martin U. 2012; Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation. 126:430–439. DOI:
10.1161/CIRCULATIONAHA.111.087684. PMID:
22767659.

11. Berninger MT, Mohajerani P, Wildgruber M, Beziere N, Kimm MA, Ma X, Haller B, Fleming MJ, Vogt S, Anton M, Imhoff AB, Ntziachristos V, Meier R, Henning TD. 2017; Detection of intramyocardially injected DiR-labeled mesenchymal stem cells by optical and optoacoustic tomography. Photoacoustics. 6:37–47. DOI:
10.1016/j.pacs.2017.04.002. PMID:
28540184. PMCID:
PMC5430154.

12. Pei Z, Zeng J, Song Y, Gao Y, Wu R, Chen Y, Li F, Li W, Zhou H, Yang Y. 2017; In vivo imaging to monitor differentiation and therapeutic effects of transplanted mesenchymal stem cells in myocardial infarction. Sci Rep. 7:6296. DOI:
10.1038/s41598-017-06571-8. PMID:
28740146. PMCID:
PMC5524783.

13. Silva AK, Wilhelm C, Kolosnjaj-Tabi J, Luciani N, Gazeau F. 2012; Cellular transfer of magnetic nanoparticles via cell microvesicles: impact on cell tracking by magnetic resonance imaging. Pharm Res. 29:1392–1403. DOI:
10.1007/s11095-012-0680-1. PMID:
22271049.

14. Zhao Y, Korolj A, Feric N, Radisic M. 2016; Human pluripotent stem cell-derived cardiomyocyte based models for cardiotoxicity and drug discovery. Expert Opin Drug Saf. 15:1455–1458. DOI:
10.1080/14740338.2016.1223624. PMID:
27560951.

15. Ban K, Bae S, Yoon YS. 2017; Current strategies and challenges for purification of cardiomyocytes derived from human pluripotent stem cells. Theranostics. 7:2067–2077. DOI:
10.7150/thno.19427. PMID:
28638487. PMCID:
PMC5479288.

16. Denning C, Borgdorff V, Crutchley J, Firth KS, George V, Kalra S, Kondrashov A, Hoang MD, Mosqueira D, Patel A, Prodanov L, Rajamohan D, Skarnes WC, Smith JG, Young LE. 2016; Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 1863(7 Pt B):1728–1748. DOI:
10.1016/j.bbamcr.2015.10.014. PMID:
26524115. PMCID:
PMC5221745.

17. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. 2014; Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 510:273–277. DOI:
10.1038/nature13233. PMID:
24776797. PMCID:
PMC4154594.

18. Goineau S, Castagné V. 2018; Electrophysiological characteristics and pharmacological sensitivity of two lines of human induced pluripotent stem cell derived cardiomyocytes coming from two different suppliers. J Pharmacol Toxicol Methods. 90:58–66. DOI:
10.1016/j.vascn.2017.12.003. PMID:
29274391.

19. Lei CL, Wang K, Clerx M, Johnstone RH, Hortigon-Vinagre MP, Zamora V, Allan A, Smith GL, Gavaghan DJ, Mirams GR, Polonchuk L. 2017; Tailoring mathematical models to stem-cell derived cardiomyocyte lines can improve predictions of drug-induced changes to their electrophysiology. Front Physiol. 8:986. DOI:
10.3389/fphys.2017.00986. PMID:
29311950. PMCID:
PMC5732978.

20. Müller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. 2006; Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 41:876–884. DOI:
10.1016/j.yjmcc.2006.07.023. PMID:
16973174.

21. Teng CJ, Luo J, Chiu RC, Shum-Tim D. 2006; Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 132:628–632. DOI:
10.1016/j.jtcvs.2006.05.034. PMID:
16935119.

22. Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. 2007; Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 25:1015–1024. DOI:
10.1038/nbt1327. PMID:
17721512.

23. Obata Y, Suzuki D, Takeoka S. 2008; Evaluation of cationic assemblies constructed with amino acid based lipids for plasmid DNA delivery. Bioconjug Chem. 19:1055–1063. DOI:
10.1021/bc700416u. PMID:
18429628.

24. Arbab AS, Yocum GT, Wilson LB, Parwana A, Jordan EK, Kalish H, Frank JA. 2004; Comparison of transfection agents in forming complexes with ferumoxides, cell labeling efficiency, and cellular viability. Mol Imaging. 3:24–32. DOI:
10.1162/153535004773861697. PMID:
15142409.

25. Diana V, Bossolasco P, Moscatelli D, Silani V, Cova L. 2013; Dose dependent side effect of superparamagnetic iron oxide nanoparticle labeling on cell motility in two fetal stem cell populations. PLoS One. 8:e78435. DOI:
10.1371/journal.pone.0078435. PMID:
24244310. PMCID:
PMC3820601.

26. Hardman R. 2006; A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect. 114:165–172. DOI:
10.1289/ehp.8284. PMID:
16451849. PMCID:
PMC1367826.

27. Brenan M, Parish CR. 1984; Intracellular fluorescent labelling of cells for analysis of lymphocyte migration. J Immunol Methods. 74:31–38. DOI:
10.1016/0022-1759(84)90364-8. PMID:
6438234.

28. Boutonnat J, Muirhead KA, Barbier M, Mousseau M, Ronot X, Seigneurin D. 1998; PKH26 probe in the study of the proliferation of chemoresistant leukemic sublines. Anticancer Res. 18:4243–4251.
31. Robertson C, Tran DD, George SC. 2013; Concise review: maturation phases of human pluripotent stem cell-derived cardiomyocytes. Stem Cells. 31:829–837. DOI:
10.1002/stem.1331. PMID:
23355363. PMCID:
PMC3749929.

32. Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ. 2016; Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays. Sci Rep. 6:24637. DOI:
10.1038/srep24637. PMID:
27097795. PMCID:
PMC4838928.

33. Giacomelli E, Bellin M, Sala L, van Meer BJ, Tertoolen LG, Orlova VV, Mummery CL. 2017; Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development. 144:1008–1017. DOI:
10.1242/dev.143438. PMID:
28279973. PMCID:
PMC5358113.

34. Jackman C, Li H, Bursac N. 2018; Long-term contractile activity and thyroid hormone supplementation produce engineered rat myocardium with adult-like structure and function. Acta Biomater. 78:98–110. DOI:
10.1016/j.actbio.2018.08.003. PMID:
30086384. PMCID:
PMC6131056.

35. Moon SH, Kang SW, Park SJ, Bae D, Kim SJ, Lee HA, Kim KS, Hong KS, Kim JS, Do JT, Byun KH, Chung HM. 2013; The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials. 34:4013–4026. DOI:
10.1016/j.biomaterials.2013.02.022. PMID:
23465823.

36. Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ. 2019; Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 10:3123. DOI:
10.1038/s41467-019-11091-2. PMID:
31311935. PMCID:
PMC6635499.

37. Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC. 2015; Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ Res. 117:720–730. DOI:
10.1161/CIRCRESAHA.115.306985. PMID:
26291556. PMCID:
PMC4679370.

38. Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. 2012; Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 489:322–325. DOI:
10.1038/nature11317. PMID:
22864415. PMCID:
PMC3443324.

39. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. 2016; Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 538:388–391. DOI:
10.1038/nature19815. PMID:
27723741.

40. Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A, Eschenhagen T. 2016; Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 8:363ra148. DOI:
10.1126/scitranslmed.aaf8781. PMID:
27807283.

41. Guo L, Abrams RM, Babiarz JE, Cohen JD, Kameoka S, Sanders MJ, Chiao E, Kolaja KL. 2011; Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci. 123:281–289. DOI:
10.1093/toxsci/kfr158. PMID:
21693436.

42. Harris K, Aylott M, Cui Y, Louttit JB, McMahon NC, Sridhar A. 2013; Comparison of electrophysiological data from human-induced pluripotent stem cell-derived cardiomyocytes to functional preclinical safety assays. Toxicol Sci. 134:412–426. DOI:
10.1093/toxsci/kft113. PMID:
23690542.

43. Keung W, Boheler KR, Li RA. 2014; Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther. 5:17. DOI:
10.1186/scrt406. PMID:
24467782. PMCID:
PMC4055054.

44. Tan SH, Ye L. 2018; Maturation of pluripotent stem cell-derived cardiomyocytes: a critical step for drug development and cell therapy. J Cardiovasc Transl Res. 11:375–392. DOI:
10.1007/s12265-018-9801-5. PMID:
29557052.

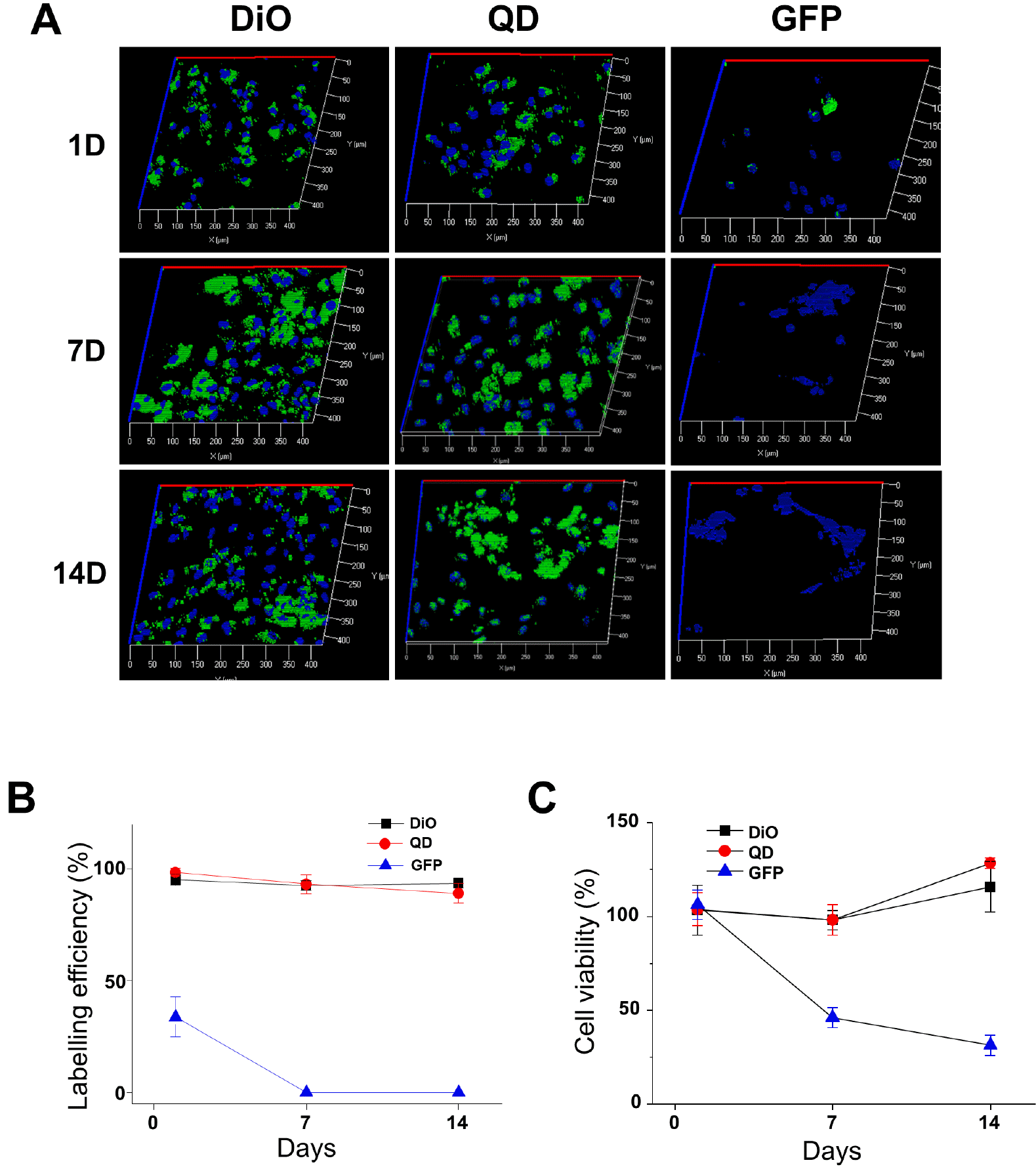
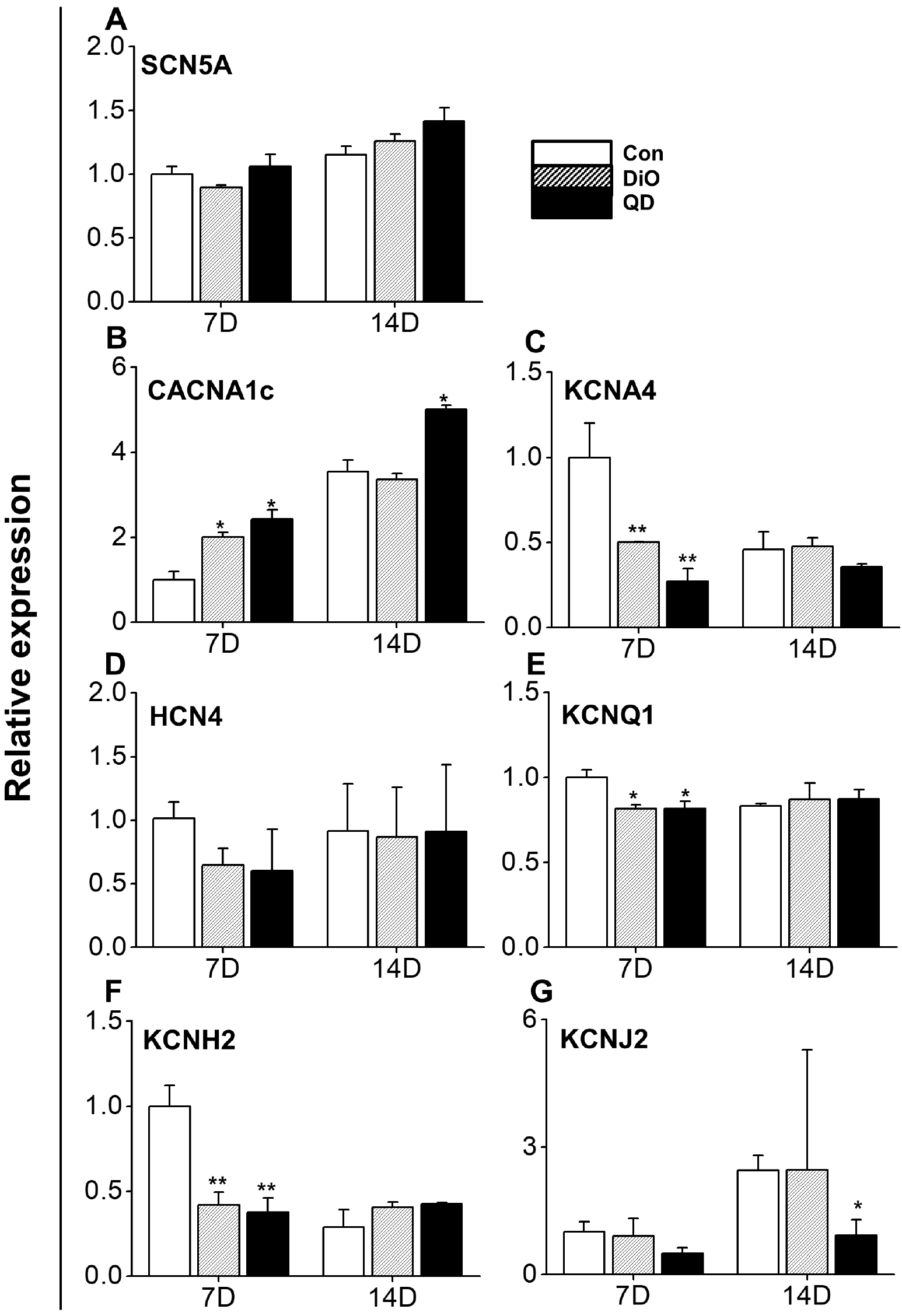
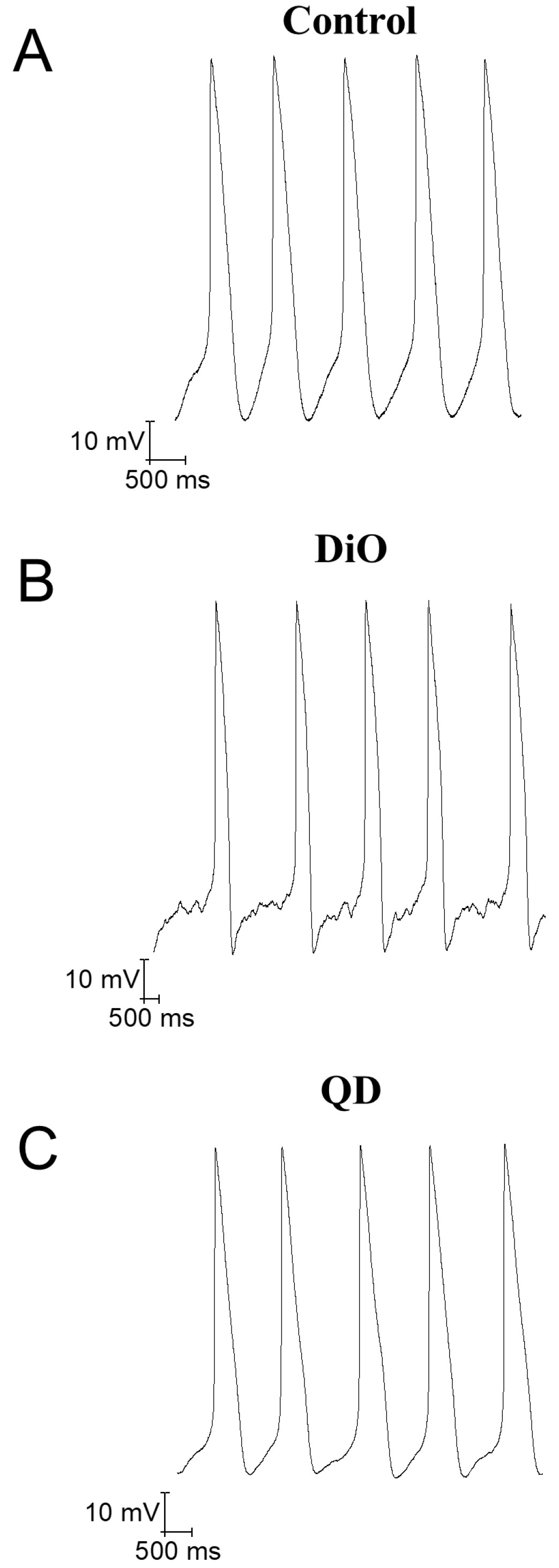
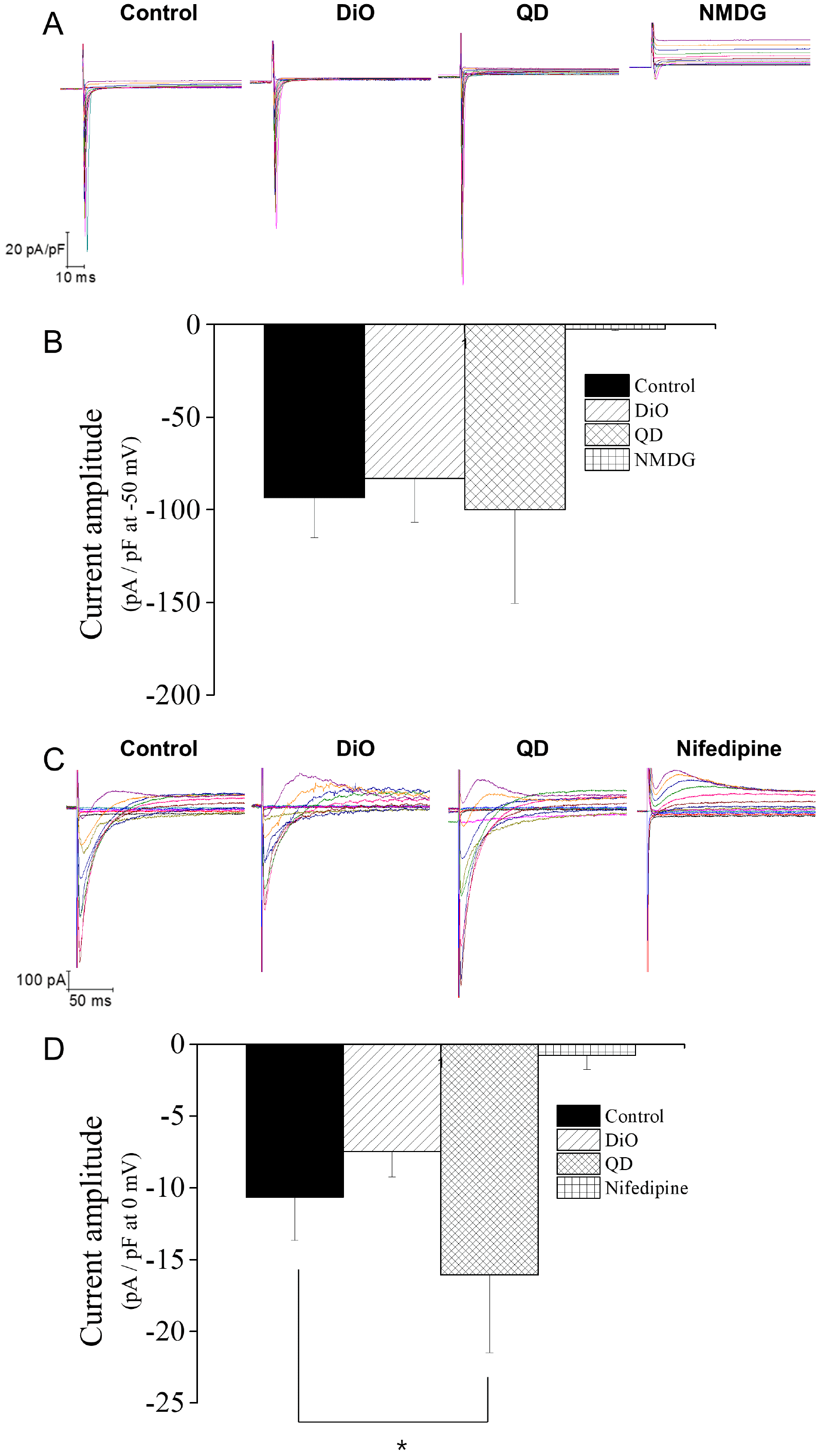




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download