1. Palis J, Yoder MC. 2001; Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 29:927–936. DOI:
10.1016/S0301-472X(01)00669-5.

2. Medvinsky A, Dzierzak E. 1996; Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 86:897–906. DOI:
10.1016/S0092-8674(00)80165-8.

3. Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HK. 2008; The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2:252–263. DOI:
10.1016/j.stem.2008.01.001. PMID:
18371450. PMCID:
PMC2888040.

4. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. DOI:
10.1126/science.282.5391.1145. PMID:
9804556.

5. Ma F, Wang D, Hanada S, Ebihara Y, Kawasaki H, Zaike Y, Heike T, Nakahata T, Tsuji K. 2007; Novel method for efficient production of multipotential hematopoietic progenitors from human embryonic stem cells. Int J Hematol. 85:371–379. DOI:
10.1532/IJH97.06203. PMID:
17562610.

6. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI:
10.1016/j.cell.2007.11.019. PMID:
18035408.

7. Ma F, Ebihara Y, Umeda K, Sakai H, Hanada S, Zhang H, Zaike Y, Tsuchida E, Nakahata T, Nakauchi H, Tsuji K. 2008; Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci U S A. 105:13087–13092. DOI:
10.1073/pnas.0802220105. PMID:
18755895. PMCID:
PMC2526552.

8. Palis J. 2016; Hematopoietic stem cell-independent hematopoiesis: emergence of erythroid, megakaryocyte, and myeloid potential in the mammalian embryo. FEBS Lett. 590:3965–3974. DOI:
10.1002/1873-3468.12459. PMID:
27790707.

10. Chen B, Teng J, Liu H, Pan X, Zhou Y, Huang S, Lai M, Bian G, Mao B, Sun W, Zhou Q, Yang S, Nakahata T, Ma F. 2017; Inducible overexpression of RUNX1b/c in human embryonic stem cells blocks early hematopoiesis from mesoderm. J Mol Cell Biol. 9:262–273. DOI:
10.1093/jmcb/mjx032. PMID:
28992293.

11. Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. 1993; p21 is a universal inhibitor of cyclin kinases. Nature. 366:701–704. DOI:
10.1038/366701a0. PMID:
8259214.

12. Ouellet S, Vigneault F, Lessard M, Leclerc S, Drouin R, Guérin SL. 2006; Transcriptional regulation of the cyclin-dependent kinase inhibitor 1A (p21) gene by NFI in proliferating human cells. Nucleic Acids Res. 34:6472–6487. DOI:
10.1093/nar/gkl861. PMID:
17130157. PMCID:
PMC1702497.

13. Stier S, Cheng T, Forkert R, Lutz C, Dombkowski DM, Zhang JL, Scadden DT. 2003; Ex vivo targeting of p21Cip1/Waf1 permits relative expansion of human hematopoietic stem cells. Blood. 102:1260–1266. DOI:
10.1182/blood-2002-10-3053. PMID:
12702511.

14. Matsumura I, Ishikawa J, Nakajima K, Oritani K, Tomiyama Y, Miyagawa J, Kato T, Miyazaki H, Matsuzawa Y, Kanakura Y. 1997; Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol Cell Biol. 17:2933–2943. DOI:
10.1128/MCB.17.5.2933. PMID:
9111365. PMCID:
PMC232145.

15. Eilken HM, Nishikawa S, Schroeder T. 2009; Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 457:896–900. DOI:
10.1038/nature07760. PMID:
19212410.

16. Mao B, Huang S, Lu X, Sun W, Zhou Y, Pan X, Yu J, Lai M, Chen B, Zhou Q, Mao S, Bian G, Zhou J, Nakahata T, Ma F. 2016; Early development of definitive erythroblasts from human pluripotent stem cells defined by expression of glycophorin A/CD235a, CD34, and CD36. Stem Cell Reports. 7:869–883. DOI:
10.1016/j.stemcr.2016.09.002. PMID:
27720903. PMCID:
PMC5106477.

17. Chang J, Sun W, Zeng J, Xue Y, Zhang Y, Pan X, Zhou Y, Lai M, Bian G, Zhou Q, Liu J, Chen B, Guo F, Ma F. 2019; Establishment of an in vitro system based on AGM-S3 co-culture for screening traditional herbal medicines that stimulate hematopoiesis. J Ethnopharmacol. 240:111938. DOI:
10.1016/j.jep.2019.111938. PMID:
31077780.

18. Zhou Y, Zhang Y, Chen B, Dong Y, Zhang Y, Mao B, Pan X, Lai M, Chen Y, Bian G, Zhou Q, Nakahata T, Zhou J, Wu M, Ma F. 2019; Overexpression of GATA2 enhances development and maintenance of human embryonic stem cell-derived hematopoietic stem cell-like progenitors. Stem Cell Reports. 13:31–47. DOI:
10.1016/j.stemcr.2019.05.007. PMID:
31178416. PMCID:
PMC6626852.

19. Palpant NJ, Wang Y, Hadland B, Zaunbrecher RJ, Redd M, Jones D, Pabon L, Jain R, Epstein J, Ruzzo WL, Zheng Y, Bernstein I, Margolin A, Murry CE. 2017; Chromatin and transcriptional analysis of mesoderm progenitor cells identifies HOPX as a regulator of primitive hematopoiesis. Cell Rep. 20:1597–1608. DOI:
10.1016/j.celrep.2017.07.067. PMID:
28813672. PMCID:
PMC5576510.

20. Yuan X, Braunstein EM, Ye Z, Liu CF, Chen G, Zou J, Cheng L, Brodsky RA. 2013; Generation of glycosylphosphatidylinositol anchor protein-deficient blood cells from human induced pluripotent stem cells. Stem Cells Transl Med. 2:819–829. DOI:
10.5966/sctm.2013-0069. PMID:
24113066. PMCID:
PMC3808197.

21. Vodyanik MA, Thomson JA, Slukvin II. 2006; Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 108:2095–2105. DOI:
10.1182/blood-2006-02-003327. PMID:
16757688. PMCID:
PMC1895535.

22. Gartel AL, Radhakrishnan SK. 2005; Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65:3980–3985. DOI:
10.1158/0008-5472.CAN-04-3995. PMID:
15899785.

23. Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. 2000; Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 287:1804–1808. DOI:
10.1126/science.287.5459.1804. PMID:
10710306.

24. Waga S, Hannon GJ, Beach D, Stillman B. 1994; The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 369:574–578. DOI:
10.1038/369574a0. PMID:
7911228.

25. Sun W, Teng J, Zeng J, Xue Y, Chang J, Zhang Y, Pan X, Zhou Y, Lai M, Bian G, Zhou Q, Liu J, Chen B, Ma F. 2019; The piggyBac-based double-inducible binary vector system: a novel universal platform for studying gene functions and interactions. Plasmid. 105:102420. DOI:
10.1016/j.plasmid.2019.102420. PMID:
31265838.

26. Xiao Y, Wang J, Song H, Zou P, Zhou D, Liu L. 2013; CD34+ cells from patients with myelodysplastic syndrome present different p21 dependent premature senescence. Leuk Res. 37:333–340. DOI:
10.1016/j.leukres.2012.11.006. PMID:
23219618.

27. Albanese P, Chagraoui J, Charon M, Cocault L, Dusanter-Fourt I, Romeo PH, Uzan G. 2002; Forced expression of p21 in GPIIb-p21 transgenic mice induces abnormalities in the proliferation of erythroid and megakaryocyte progenitors and primitive hematopoietic cells. Exp Hematol. 30:1263–1272. DOI:
10.1016/S0301-472X(02)00933-5.

28. Fortunel NO, Hatzfeld A, Hatzfeld JA. 2000; Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 96:2022–2036. DOI:
10.1182/blood.V96.6.2022. PMID:
10979943.

29. Cheng T, Shen H, Rodrigues N, Stier S, Scadden DT. 2001; Transforming growth factor beta 1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21(Cip1/Waf1) or p27(Kip1). Blood. 98:3643–3649. DOI:
10.1182/blood.V98.13.3643. PMID:
11739168.

31. Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. 2003; Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell. 113:301–314. DOI:
10.1016/S0092-8674(03)00308-8. PMID:
12732139.
32. Dutto I, Tillhon M, Cazzalini O, Stivala LA, Prosperi E. 2015; Biology of the cell cycle inhibitor p21(CDKN1A): molecular mechanisms and relevance in chemical toxicology. Arch Toxicol. 89:155–178. DOI:
10.1007/s00204-014-1430-4. PMID:
25514883.

33. Warbrick E, Lane DP, Glover DM, Cox LS. 1995; A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr Biol. 5:275–282. DOI:
10.1016/S0960-9822(95)00058-3.

34. Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. 1996; Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 16:4673–4682. DOI:
10.1128/MCB.16.9.4673. PMID:
8756624. PMCID:
PMC231467.

35. Oku T, Ikeda S, Sasaki H, Fukuda K, Morioka H, Ohtsuka E, Yoshikawa H, Tsurimoto T. 1998; Functional sites of human PCNA which interact with p21 (Cip1/Waf1), DNA polymerase delta and replication factor C. Genes Cells. 3:357–369. DOI:
10.1046/j.1365-2443.1998.00199.x. PMID:
9734782.

36. Gottifredi V, McKinney K, Poyurovsky MV, Prives C. 2004; Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J Biol Chem. 279:5802–5810. DOI:
10.1074/jbc.M310373200. PMID:
14597617.

37. Cazzalini O, Perucca P, Riva F, Stivala LA, Bianchi L, Vannini V, Ducommun B, Prosperi E. 2003; p21CDKN1A does not interfere with loading of PCNA at DNA replication sites, but inhibits subsequent binding of DNA polymerase delta at the G1/S phase transition. Cell Cycle. 2:596–603. DOI:
10.4161/cc.2.6.502. PMID:
14504476.
38. Smits VA, Klompmaker R, Vallenius T, Rijksen G, Mäkela TP, Medema RH. 2000; p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 275:30638–30643. DOI:
10.1074/jbc.M005437200. PMID:
10913154.

39. Charrier-Savournin FB, Château MT, Gire V, Sedivy J, Piette J, Dulic V. 2004; p21-Mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol Biol Cell. 15:3965–3976. DOI:
10.1091/mbc.e03-12-0871. PMID:
15181148. PMCID:
PMC515331.

40. Gillis LD, Leidal AM, Hill R, Lee PW. 2009; p21Cip1/WAF1 mediates cyclin B1 degradation in response to DNA damage. Cell Cycle. 8:253–256. DOI:
10.4161/cc.8.2.7550. PMID:
19158493.

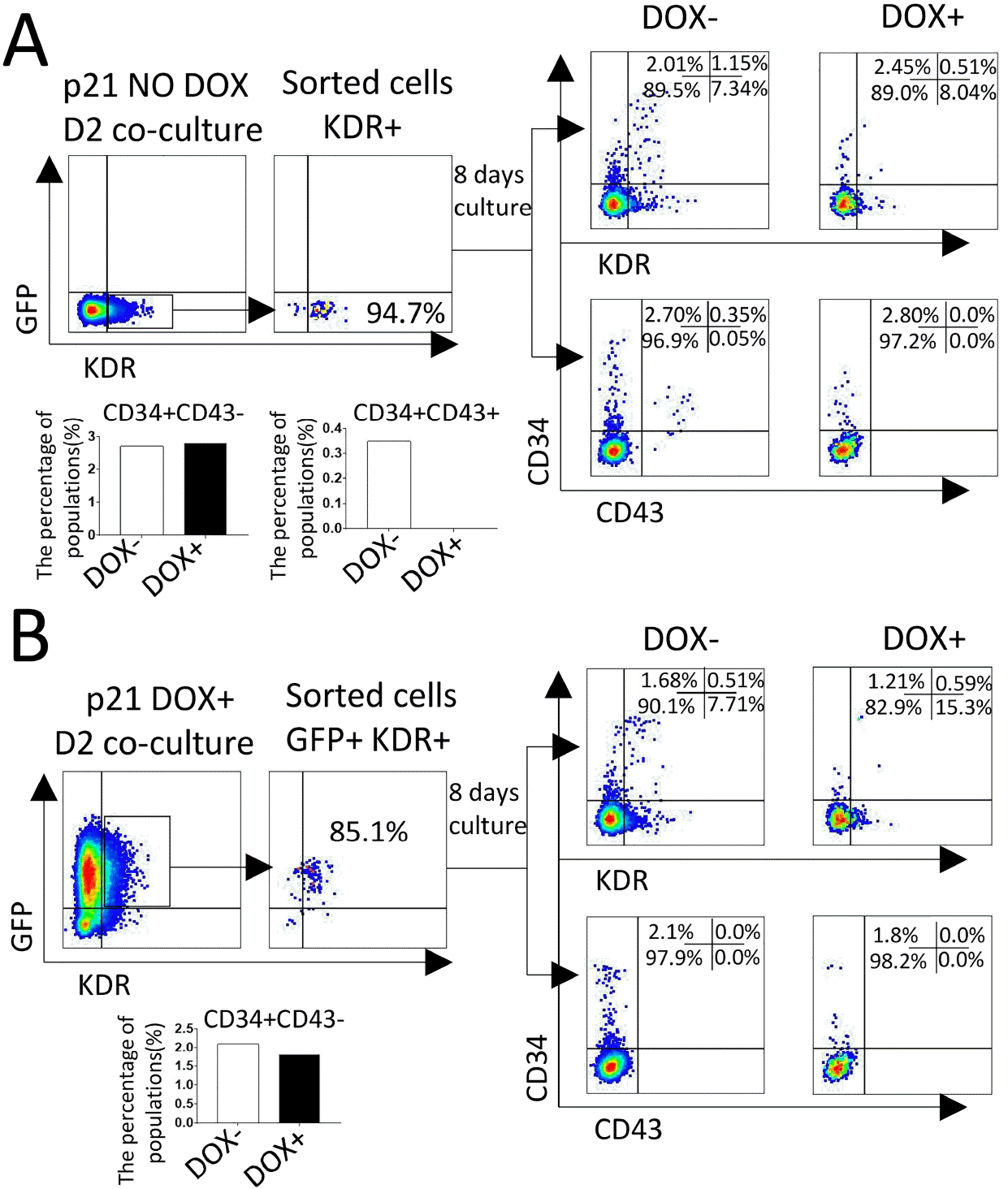
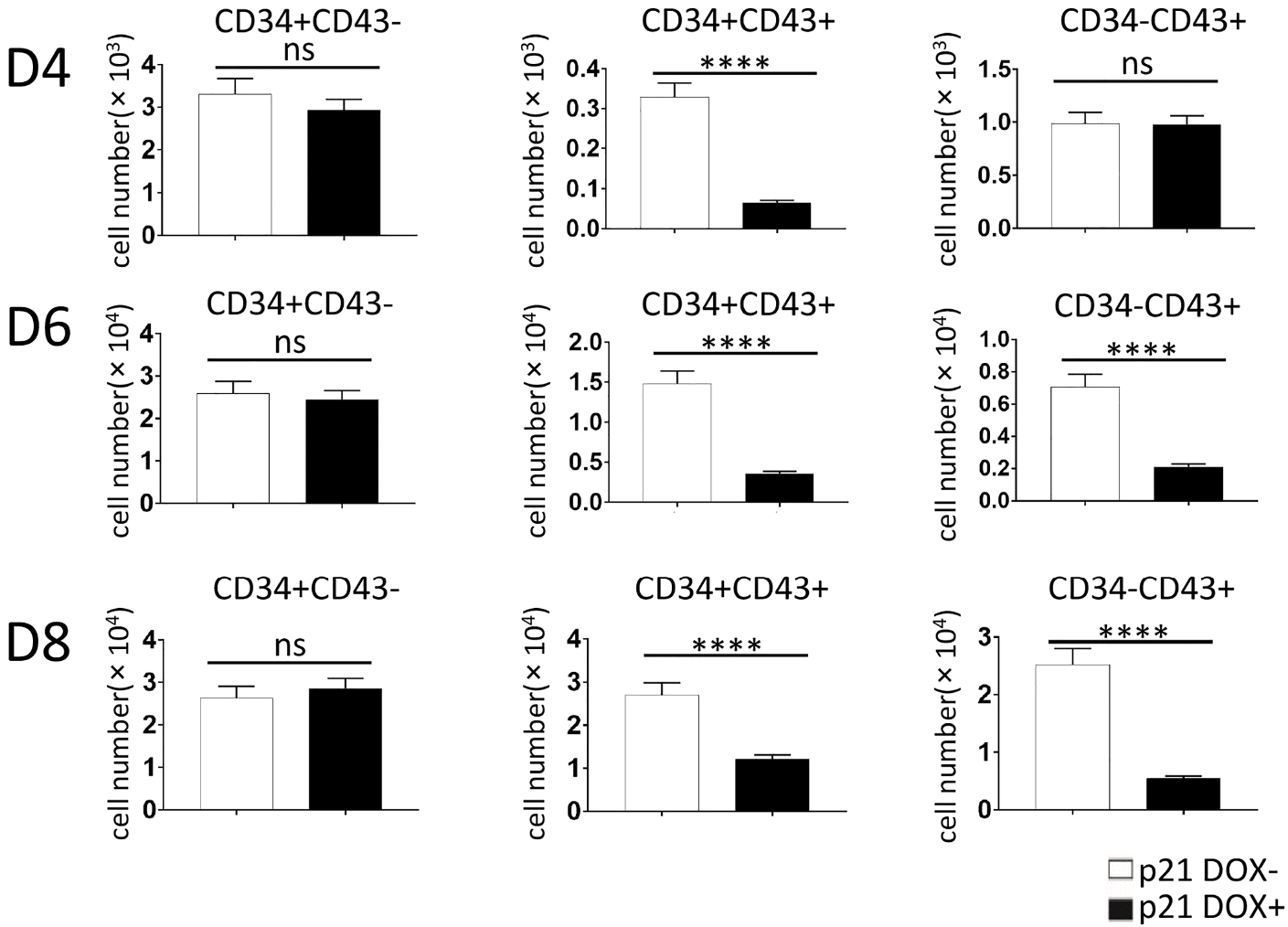
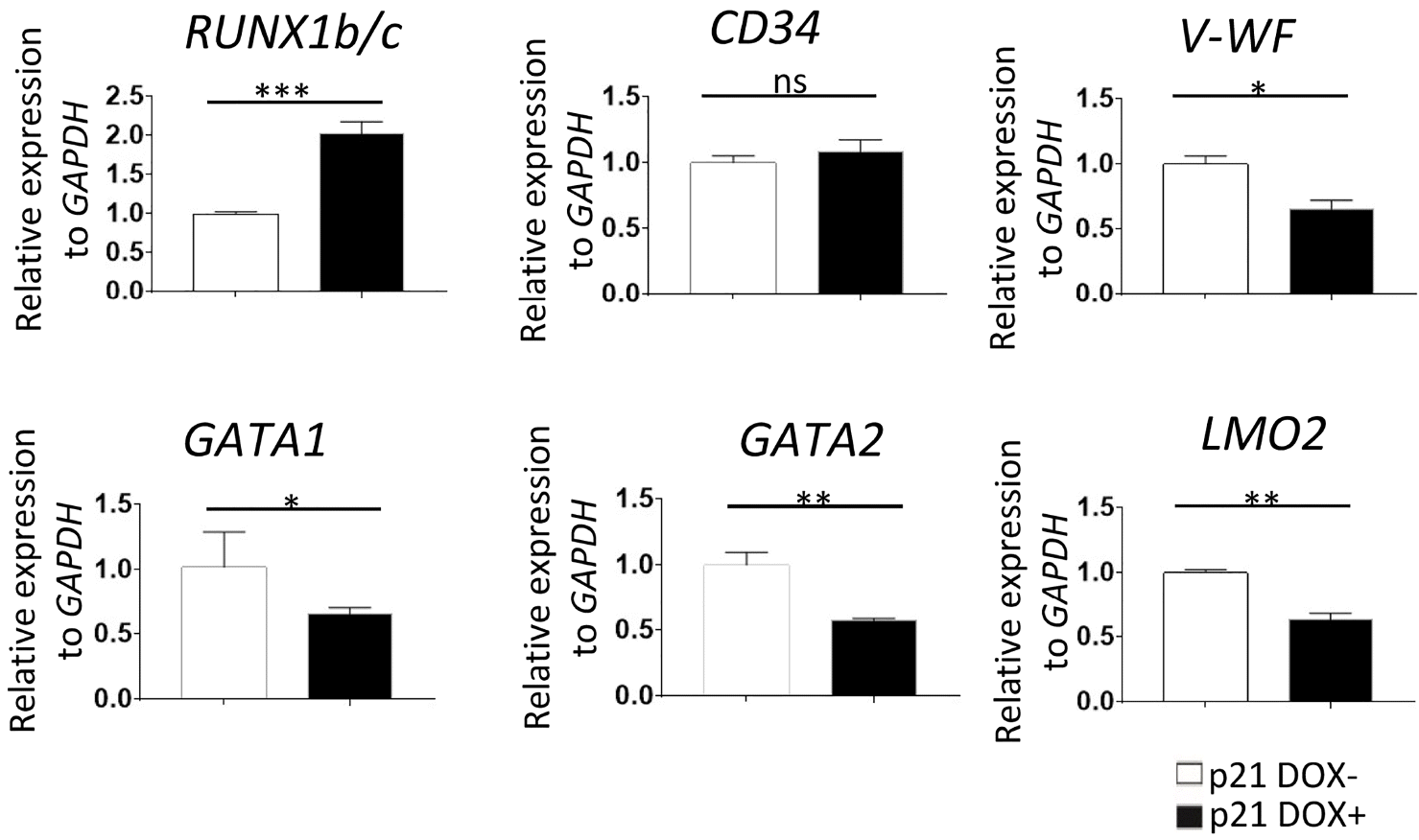
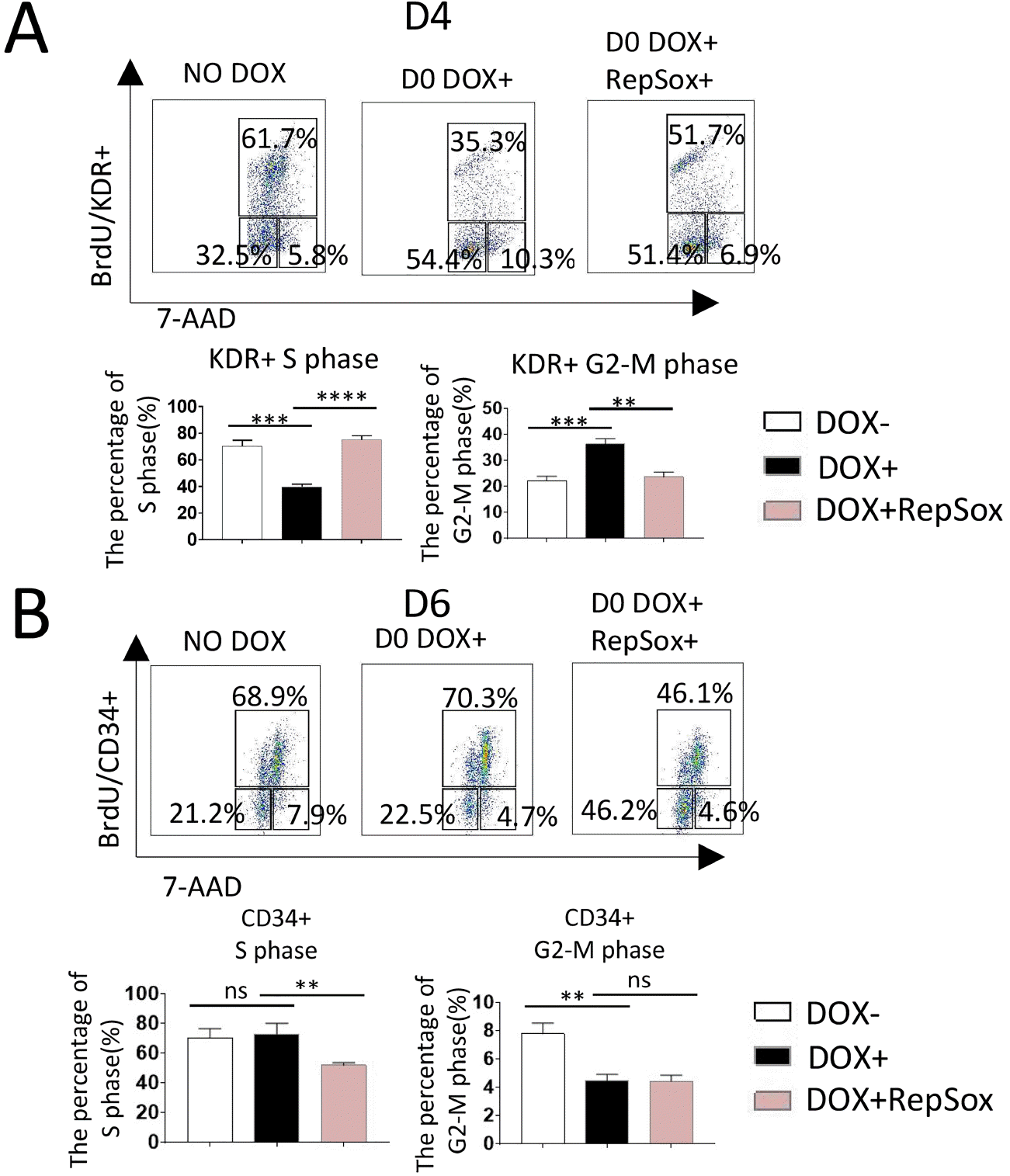




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download