Abstract
Osteoarthritis (OA) is the most common type of arthritis and causes a significant deterioration in patients’ quality of life. The high prevalence of OA as well as the current lack of disease-modifying drugs led to a rise in regenerative medicine efforts. The hope is that this will provide a treatment modality with the ability to alter the course of OA via structural modifications of damaged articular cartilage (AC). Regenerative therapy in OA starts with the concept that administered cells may engraft to a lesion site and differentiate into chondrocytes. However, recent studies show that cells, particularly when injected in suspension, rapidly undergo apoptosis after exerting a transient paracrine effect. If the injected stem cells do not lead to structural improvements of a diseased joint, the high cost of cell therapy for OA cannot be justified, particularly when compared with other injection therapeutics such as corticosteroids and hyaluronic acid. Long-term survival of implanted cells that offer prolonged paracrine effects or possible engraftment is essential for a successful cell therapy that will offer durable structural improvements. In this perspective review, the history and current status of regenerative therapy in OA are summarized along with the conceptual strategy and future directionsfor a successful regenerative therapy that can provide structural modifications in OA.
Go to : 
Regenerative therapies in osteoarthritis (OA) began with the concept that administered cells may engraft to lesion sites and differentiate into chondrocytes. However, recent studies have shown that cells, particularly when injected in suspension, undergo apoptosis after exerting a transient paracrine effect (1, 2). While paracrine action may include the mobilization of endogenous stem cells that contribute to the formation of regenerative neo-cartilage, the anti-inflammatory effect of innate immunity has been shown to be a more prominent paracrine action than the chondrogenic effect (3, 4).
If injected stem cells disappear after exerting a brief anti-inflammatory effect and do not contribute to structural improvement, i.e., regeneration of articular cartilage (AC), the high cost of cell therapy for OA cannot be justified, particularly when compared with other injection therapeutics such as corticosteroids and hyaluronic acid. Long-term survival of implanted cells that offer a prolonged paracrine effect or possibly engraftment is essential for a successful cell therapy in OA that will offer durable structural improvements.
Go to : 
OA is the most common type of arthritis. It ischaracterized by loss of AC, subchondral sclerosis, osteophyte formation, and, ultimately, joint destruction (5). Quality of life significantly deteriorates in OA patients with increased pain and loss of joint function (6). The current treatments for early OA are weight reduction, exercise, braces, nonsteroidal anti-inflammatory drugs, and intra-articular (IA) injections of glucocorticoid or hyaluronic acid (HA) in advanced cases, joint replacement has been the mainstay treatment for decades (7, 8). Pharmaceutical treatment provides alleviation of symptoms such as pain and inflammation. There are currently no disease-modifying drugs that alter the natural history of OA and offer structural improvements in damaged AC. Joint replacements are frequently associated with serious life-threatening complications, including thromboembolism (9) and periprosthetic infection (10). The high prevalence of OA as well as the current lack of disease-modifying drugs has led researchers and physicians to explore regenerative medicine as a possible treatment modality that may alter the course of OA via structural modification of damaged AC.
Go to : 
Cell therapy for cartilage regeneration was first based on the belief that implanted cells regenerate damaged AC, thus bringing about structural modification of a diseased joint, and might eventually replace pharmaceutical and surgical treatments for OA. Autologous chondrocytes were the first cell candidates. However, shortcomings of the treatment, such as loss of chondrocyte phenotypes andadditional morbidity associated with the process of harvesting chondrocytes, ledresearchers to consider other sources of therapeutic cells, mostly mesenchymal stem cells (MSC) (1, 2, 11).
Early experimental studies for stem cell-based cartilage regeneration focused on the induction of chondrogenic differentiation from stem cells. A number of studies attempted to define the appropriate combination of growth factors or gene transfers that would induce chondrogenesis from stem cells (12-18). Others attempted to apply biomechanical stimuli to enhance chondrogenic induction from stem cells (19, 20).
Early induction of hypertrophic markers such as type 10 collagen and runx-2 in MSC chondrogenesis revealed a prominent difference from articular chondrocytes, which never express these markers (17, 18). As a corollary,researchers made great efforts to devise methods of suppressing hypertrophy in MSC chondrogenesis. However, if IA-administered cells do not survive long enough to differentiate into articular chondrocytes, these efforts would be useless, not producing any improvement.
Go to : 
Notably, the inflammatory environment of an OA joint provides generally inhospitable conditions for tissue regeneration. Most IA-administered stem cells undergo rapid apoptosis. The survival of cells can vary from 3 days to several weeks depending on the mode of administration and the IA environment (1, 2). These cells secrete several paracrine factors before undergoing apoptosis. These factors were found to possess predominantly anti-inflammatory and immunosuppressive actions rather than chondrogenic effects. Murphy et al. demonstrated the absence of IA-injected MSCs 1 week after injection in an ovine model (21). Kolon Life Science developed cell therapeutics consisting of TGF-b-transduced chondrocytes. Those cells were observed to completely disappear from the injected joints within 2 weeks (22). When cells were focally implanted rather than injected, longer-term survival of stem cells was reported, as seen in the application of CartistemⓇ in advanced OA (23).
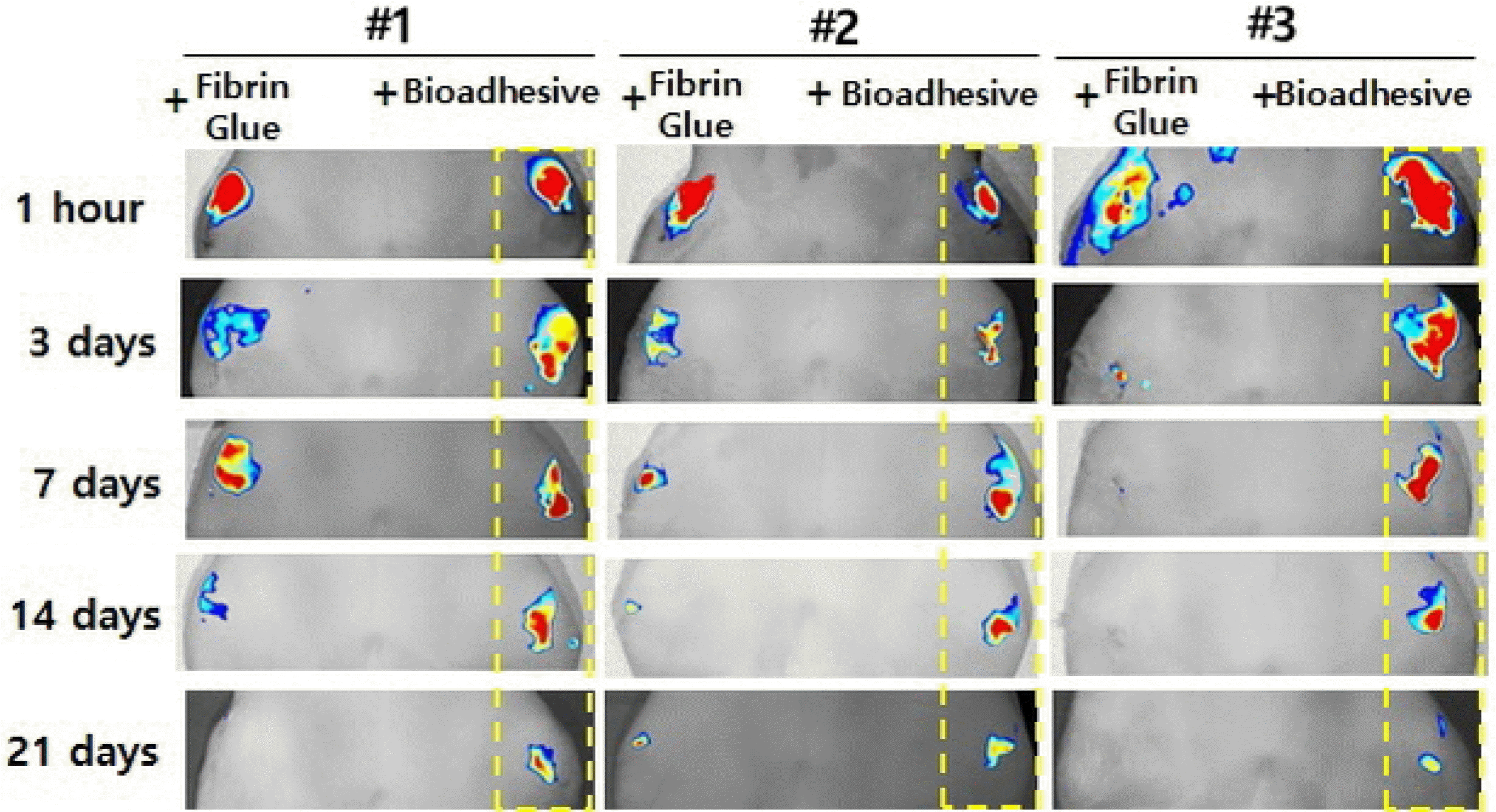
If a stem cell therapy aims to achieve structural improvementsto lesion sites by regeneration of AC, prolonged survival of implanted stem cells that could exert prolonged paracrine effectsand/or engraftment with chondrogenic differentiation are mandatory requirements. The author’s preliminary results show that adipose stem cells (ASCs) in spheroid form survive longer post-IA injection than do ASCs that are injected in a free single cell suspension. These findings mean that some communication/interaction between cells can promote IA cell survival, as in monolayer cell culture, in which a certain concentration of cells is necessary for survival. Also, ASCs that were fixed on the focal chondral defect using a strong bioadhesive (mussel adhesive protein) showed longer-term survival thanthose immobilized using fibrin glue. These results indicate that stem cells can survive longer when forced to stay at the site of implantation (Fig. 1).
Go to : 
Cellular aging naturally reduces the survival of stem cells. Oxidative stress promotes cellular aging. In aged MSCs, superoxide dismutase (SOD), an important antioxidant enzyme, is decreased (24). Conversely, hypoxic states or antioxidants such as ascorbic acid, N-acetylcystein, erythropoietin, and sulforaphane increase stem cell survival (25, 26). The PI3K/AKT/mTOR/FOXO3 pathways play a significant role in modulating oxidative stress. An mTOR inhibitor, rapamycin, suppresses the production of ROS (27, 28). Aged MSCs secrete molecules that aggravate inflammation, including leptin, TGF-A, interleukin-8, eoxtaxin, interferon-γ, VCAM1, interferon-β, interleukin-4, and monocyte chemotactic protein-1 (MCP1) (29).
A vast wealth of knowledge has been obtained from the study of aging and survival of stem cells. Pre-treatment with substances known to promote cell survival may enhance IA survival of administered stem cell therapeutics.
Go to : 
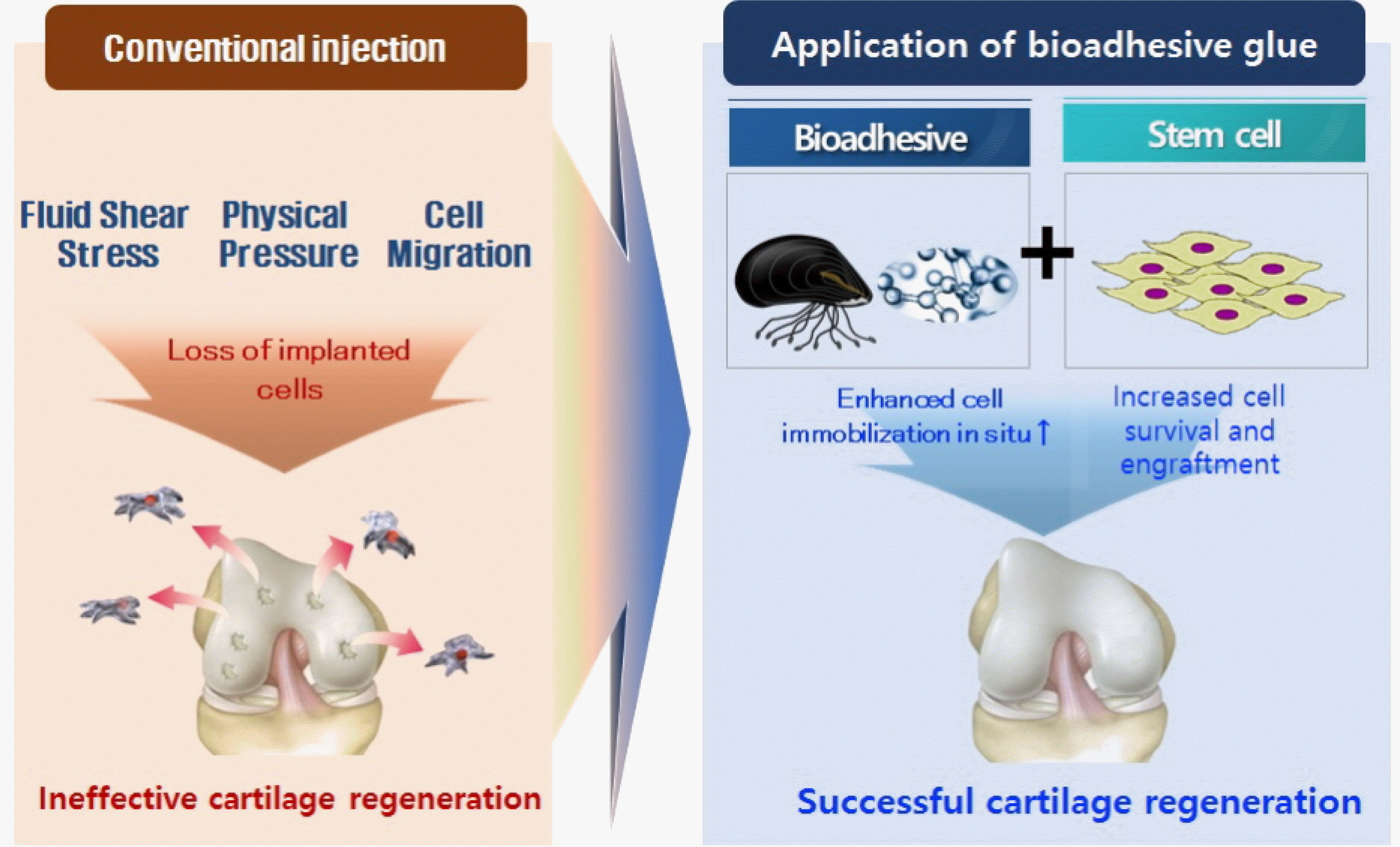
When cell therapy was first reported to treat cartilage defects and osteoarthritis, an optimistic view prevailed which held that implanted cells could be incorporated into defects and regenerate AC. The main focus was on how to ensure that the implanted stem cells possess the full properties of articular chondrocytes. As it became evident that almost all IA-administered stem cells rapidly undergo apoptosis and that theirprincipal mode of action is paracrine, two strategies rose up to help enhance the effects of cell therapeutics: 1) enhance the survival of stem cells by pretreatment with factors known to promote cell survival or by administration of stem cells in physical states that favor longer-term survival, such as the spheroid form or encapsulated in a hydrogel, and 2) apply stem cells in a high concentration and prevent disperal into the joint using a bioadhesive (Fig. 2).
Given that the mechanisms underlying necrosis versus survival of implanted stem cells are not well-established, future studies should focus on how the fate of IA-administered stem cells is affected by factors such as the physical status of the cells, mode of implantation, and adjuvant biomaterials.
Go to : 
Acknowledgments
This review was supported by a grant of the National Research Foundation of Korea (NRF-2020R1A2C2008266).
Go to : 
References
1. Im GI. 2019; Perspective on intra-articular injection cell therapy for osteoarthritis treatment. Tissue Eng Regen Med. 16:357–363. DOI: 10.1007/s13770-018-00176-6. PMID: 31413940. PMCID: PMC6675827.

2. Im GI. 2018; Tissue engineering in osteoarthritis: current status and prospect of mesenchymal stem cell therapy. BioDrugs. 32:183–192. DOI: 10.1007/s40259-018-0276-3. PMID: 29704190.

3. ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PL. 2012; Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 64:3604–3613. DOI: 10.1002/art.34626. PMID: 22961401.

4. von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. 2012; Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 30:1575–1578. DOI: 10.1002/stem.1118. PMID: 22553154.

5. Goldring MB, Goldring SR. 2010; Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 1192:230–237. DOI: 10.1111/j.1749-6632.2009.05240.x. PMID: 20392241.

6. Murray CJL, Lóez AD. 1996. The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Harvard School of Public Health;Estados Unidos:
7. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology. 2012; 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 64:465–474. DOI: 10.1002/acr.21596. PMID: 22563589.

8. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M. Standing Committee for International Clinical Studies Including Therapeutic Trials ESCISIT. 2003; EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committeefor International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 62:1145–1155. DOI: 10.1136/ard.2003.011742. PMID: 14644851. PMCID: PMC1754382.
9. White RH, Romano PS, Zhou H, Rodrigo J, Bargar W. 1998; Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med. 158:1525–1531. DOI: 10.1001/archinte.158.14.1525. PMID: 9679793.

10. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. 2012; Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 27(8 Suppl):61–65.e1. DOI: 10.1016/j.arth.2012.02.022. PMID: 22554729.

11. Chang YH, Liu HW, Wu KC, Ding DC. 2016; Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 25:937–950. DOI: 10.3727/096368915X690288. PMID: 26688464.

12. Im GI, Jung NH, Tae SK. 2006; Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng. 12:527–536. DOI: 10.1089/ten.2006.12.527. PMID: 16579686.

13. Kim HJ, Im GI. 2009; Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 27:612–619. DOI: 10.1002/jor.20766. PMID: 18985688.

14. Kim HJ, Im GI. 2009; Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue EngPart A. 15:1543–1551. DOI: 10.1089/ten.tea.2008.0368. PMID: 19072523.

15. Im GI, Kim HJ. 2011; Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthritis Cartilage. 19:449–457. DOI: 10.1016/j.joca.2011.01.005. PMID: 21251990.

16. Kim YJ, Kim HJ, Im GI. 2008; PTHrP promotes chondrogenesisand suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 373:104–108. DOI: 10.1016/j.bbrc.2008.05.183. PMID: 18554504.

17. Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. 2003; Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 48:418–429. DOI: 10.1002/art.10767. PMID: 12571852.

18. Mueller MB, Tuan RS. 2008; Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 58:1377–1388. DOI: 10.1002/art.23370. PMID: 18438858. PMCID: PMC3612425.

19. Behrendt P, Ladner Y, Stoddart MJ, Lippross S, Alini M, Eglin D, Armiento AR. 2020; Articular joint-simulating mechanical load activates endogenous TGF-β in a highly cellularized bioadhesive hydrogel for cartilage repair. Am J Sports Med. 48:210–221. DOI: 10.1177/0363546519887909. PMID: 31877102.

20. Fahy N, Alini M, Stoddart MJ. 2018; Mechanical stimulation of mesenchymal stem cells: implications for cartilage tissue engineering. J Orthop Res. 36:52–63. DOI: 10.1002/jor.23670. PMID: 28763118.

21. Murphy JM, Fink DJ, Hunziker EB, Barry FP. 2003; Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 48:3464–3474. DOI: 10.1002/art.11365. PMID: 14673997.

22. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. 2017; Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 6:613–621. DOI: 10.5966/sctm.2016-0157. PMID: 28191757. PMCID: PMC5442809.

23. Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, Lee JH, Yoo JD, Bin SI, Choi CH, Kyung HS, Lee MC. 2018; A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev. 29:48–59. DOI: 10.1089/humc.2017.249. PMID: 29641281.

24. Milani P, Ambrosi G, Gammoh O, Blandini F, Cereda C. 2013; SOD1 and DJ-1 converge at Nrf2 pathway: a clue for antioxidant therapeutic potential in neurodegeneration. Oxid Med Cell Longev. 2013:836760. DOI: 10.1155/2013/836760. PMID: 23983902. PMCID: PMC3745953.

25. Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. 2005; Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 14:92–102. DOI: 10.1089/scd.2005.14.92. PMID: 15725748.

26. Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park JK. 2008; Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J BiosciBioeng. 105:586–594. DOI: 10.1263/jbb.105.586. PMID: 18640597.

27. Gharibi B, Farzadi S, Ghuman M, Hughes FJ. 2014; Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells. 32:2256–2266. DOI: 10.1002/stem.1709. PMID: 24659476.

28. Xu J, Qian J, Xie X, Lin L, Zou Y, Fu M, Huang Z, Zhang G, Su Y, Ge J. 2012; High density lipoprotein protects mesenchymal stem cells from oxidative stress-induced apoptosis via activation of the PI3K/Akt pathway and suppression of reactive oxygen species. Int J Mol Sci. 13:17104–17120. DOI: 10.3390/ijms131217104. PMID: 23443132. PMCID: PMC3546741.

29. Turinetto V, Vitale E, Giachino C. 2016; Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 17:E1164. DOI: 10.3390/ijms17071164. PMID: 27447618. PMCID: PMC4964536.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download