Introduction
Materials and Methods
PDMSC isolation, colonization, and maintenance
Preparation of different hyaluronan culture conditions
In vitro differentiation induction
Stem cell marker analysis
Generation time and cell growth curve
Mitochondrial localization and measurement of mitochondrial mass
Determination of relative mtDNA copy number
Measurement of intracellular ATP content
Measurement of lactate production
Measurement of intracellular reactive oxygen species
SILAC isotope labeling of PDMSC and LC-MS/MS-based quantitative proteomics
Statistical analysis
Results
PDMSC increases the proliferation rate in low-concentration medium supplemented hyaluronan but reduces its proliferation in high-concentrated hyaluronan and hyaluronan-coated surfaces
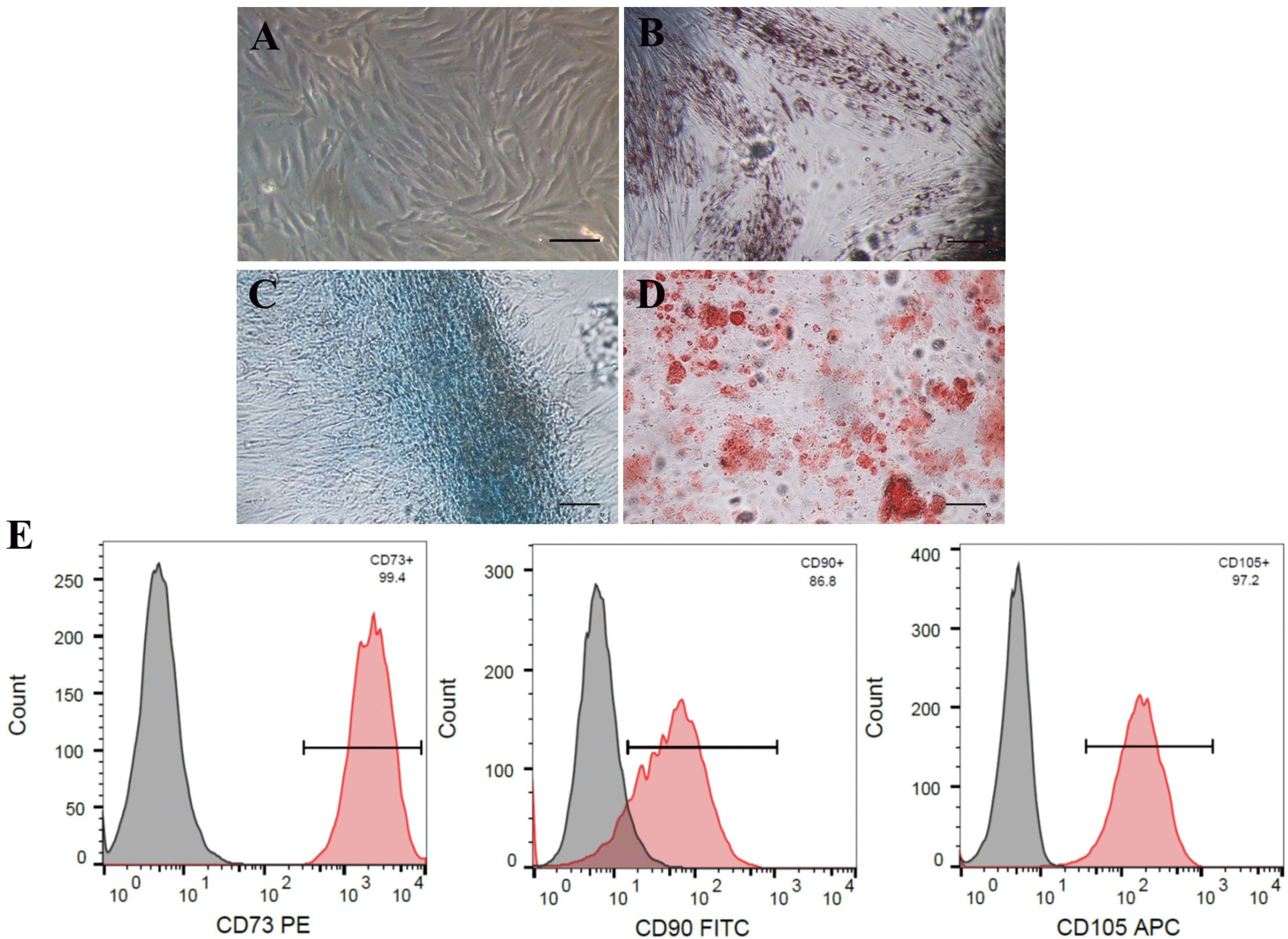 | Fig. 1PDMSCs possess plastic adherence characteristics, differentiate into MSC specific lineages, and express MSC CD Markers. PDMSCs cultured on control surface contained (A) fibroblastic morphology and plastic adherence characteristic; were assayed for differentiation ability by 32-day culture in (B) adipogenesis, (C) chondrogenesis, and (D) osteogenesis differentiation medium; and (E) positively express the MSC CD markers CD73, CD90, and CD105. Images obtained after oil red, alcian blue, and alizarin red, respectively. PE: Phycoerythrin, FITC: Fluorescein Isothiocyanate, APC: Allophycocyanin. Scale bar: 100 μm. |
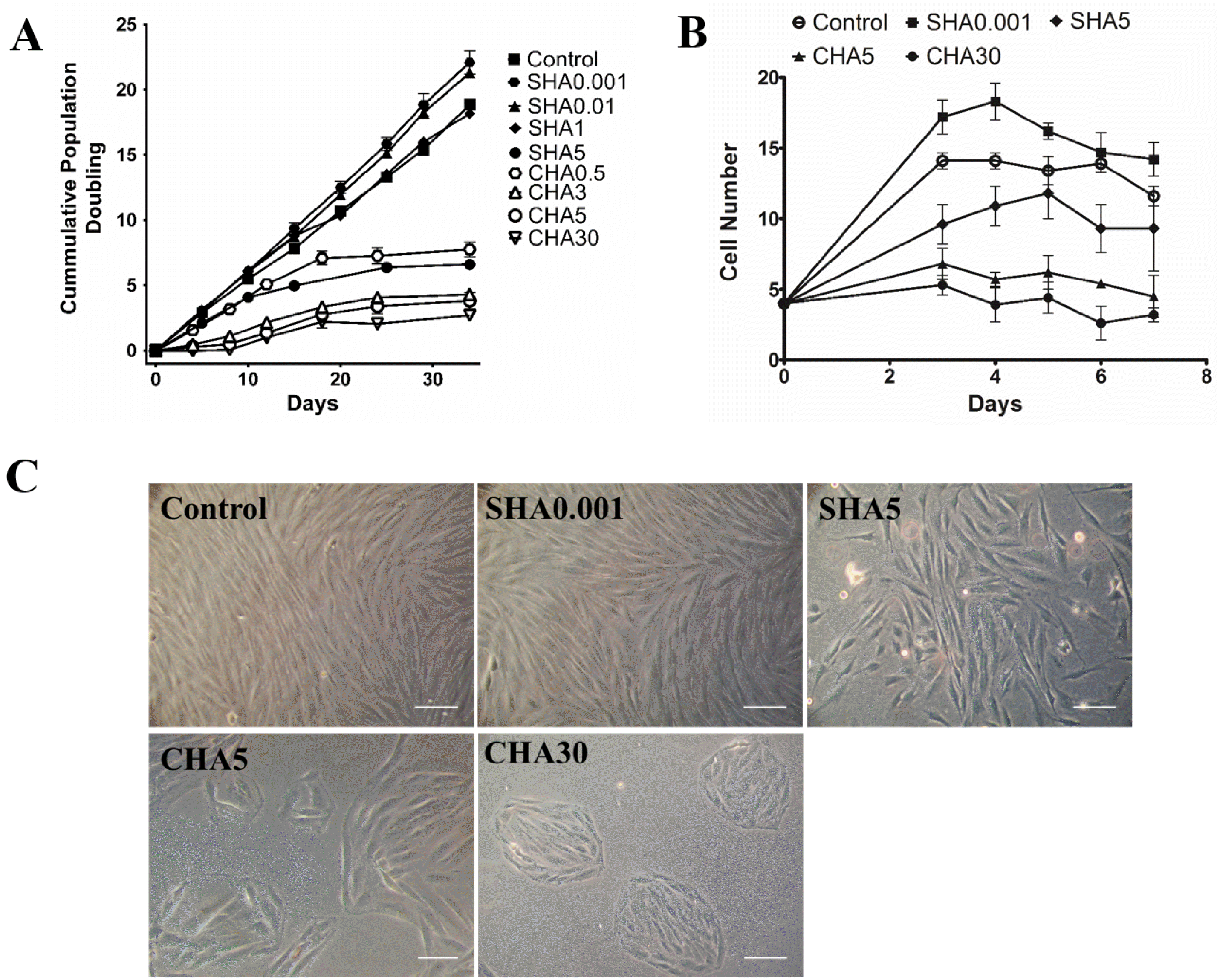 | Fig. 2Different hyaluronan substratum exerted changes in PDMSCs cellular proliferation and morphology. (A) Cumulative population doubling of PDMSCs cultured for 34 days on control, medium supplemented hyaluronan (SHA 0.001, 0.01, 1, and 5 mg/ml), or coated hyaluronan (CHA 0.5, 3, 5, 30 μg/cm2). The drawing represents the cumulative population doublings with time where each dot represents one passage approximately every 4 days. Cumulative population doubling is expressed as mean±SD of at least triplicates; (B) cell growth curve and (C) morphological changes of PDMSCs cultured for 5 days in control, SHA 0.001 and 5 mg/ml; or CHA 5 and 30 μg/cm2. Scale bar: 100 μm. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan. Three independent experiments were performed in control and experimental groups and data are represented in mean±SD. |
Different hyaluronan supplementation methods altered mitochondrial mass and mitochondrial DNA copy number of PDMSCs
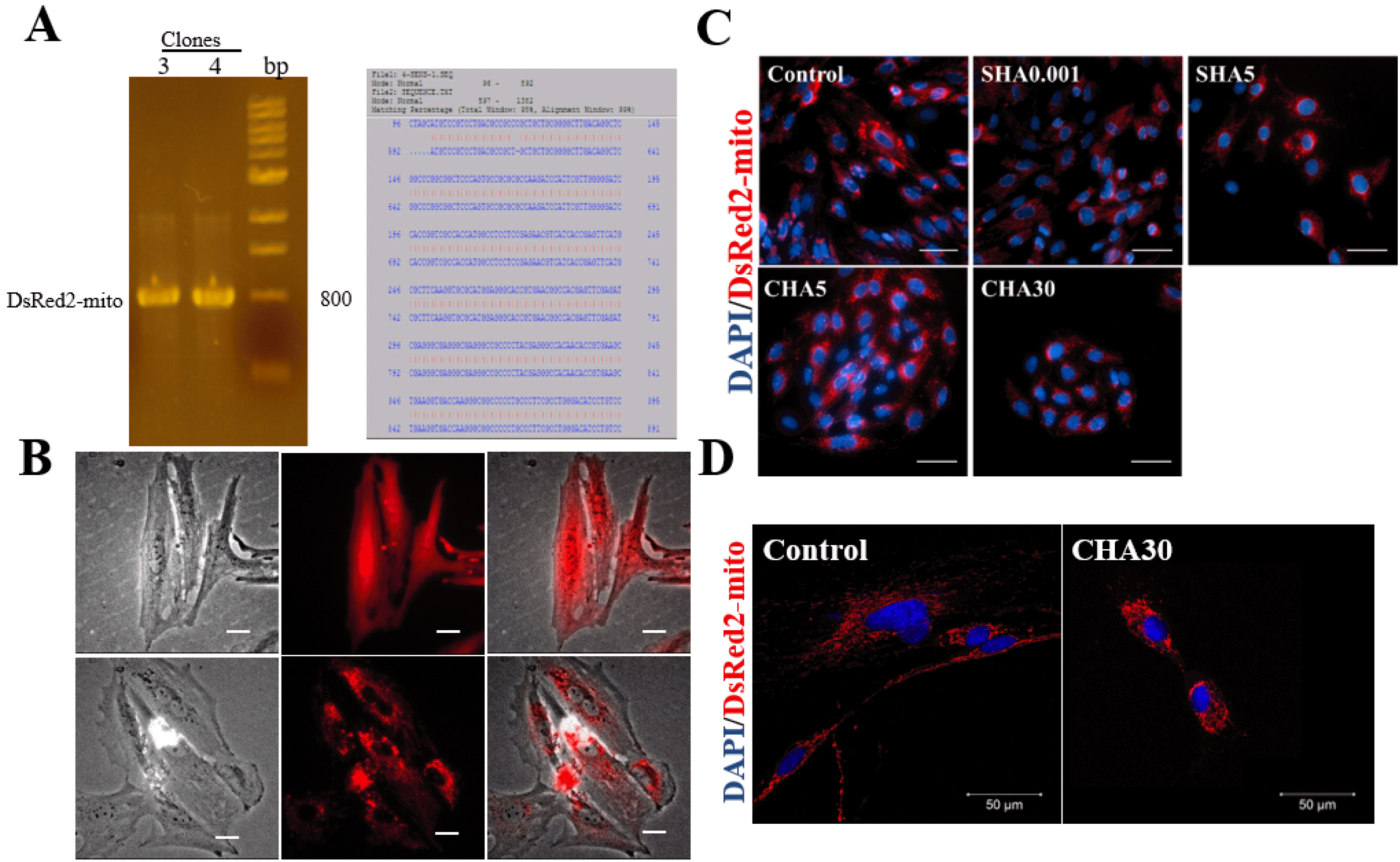 | Fig. 3Different hyaluronan substratum exerted changes in mitochondrial distribution of PDMSCs. Successful clones for lentivector pAS3W-DsRed2-mito after ligation of the vectors pLKOAS3w.puro and pDsRed2-mito. (A) PCR gels showed successful clone band at 800 bp; and sequence alignment of 99% from restriction enzyme cutting sites of NheI at 96 bp and PmeI at 892 bp. (B) Transfection of PDMSCs with pAS3W-DsRed2-mito enabled red fluorescence labelling of mitochondria specifically. Lentivector containing DsRed-mito showed fluorescence punctuated patterns when transfected into PDMSCs, whereas lentivector with only DsRed showed a diffused fluorescence pattern; (C) mitochondrial localization of PDMSCs cultured for 5 days on control surfaces, SHA 0.001 and SHA 5 mg/ml or CHA 5 and 30 μg/cm2. (D) Confocal images of mitochondrial distribution in hyaluronan-supplemented PDMSCs. Red fluorescence represents mitochondria through PDMSCs transfected with expression plasmid pAS3W-DsRed2-mito; blue fluorescence represents nucleus through DAPI staining. Scale bar: 100 μm. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, DAPI: 4’,6-diamidino-2-phenylindole. |
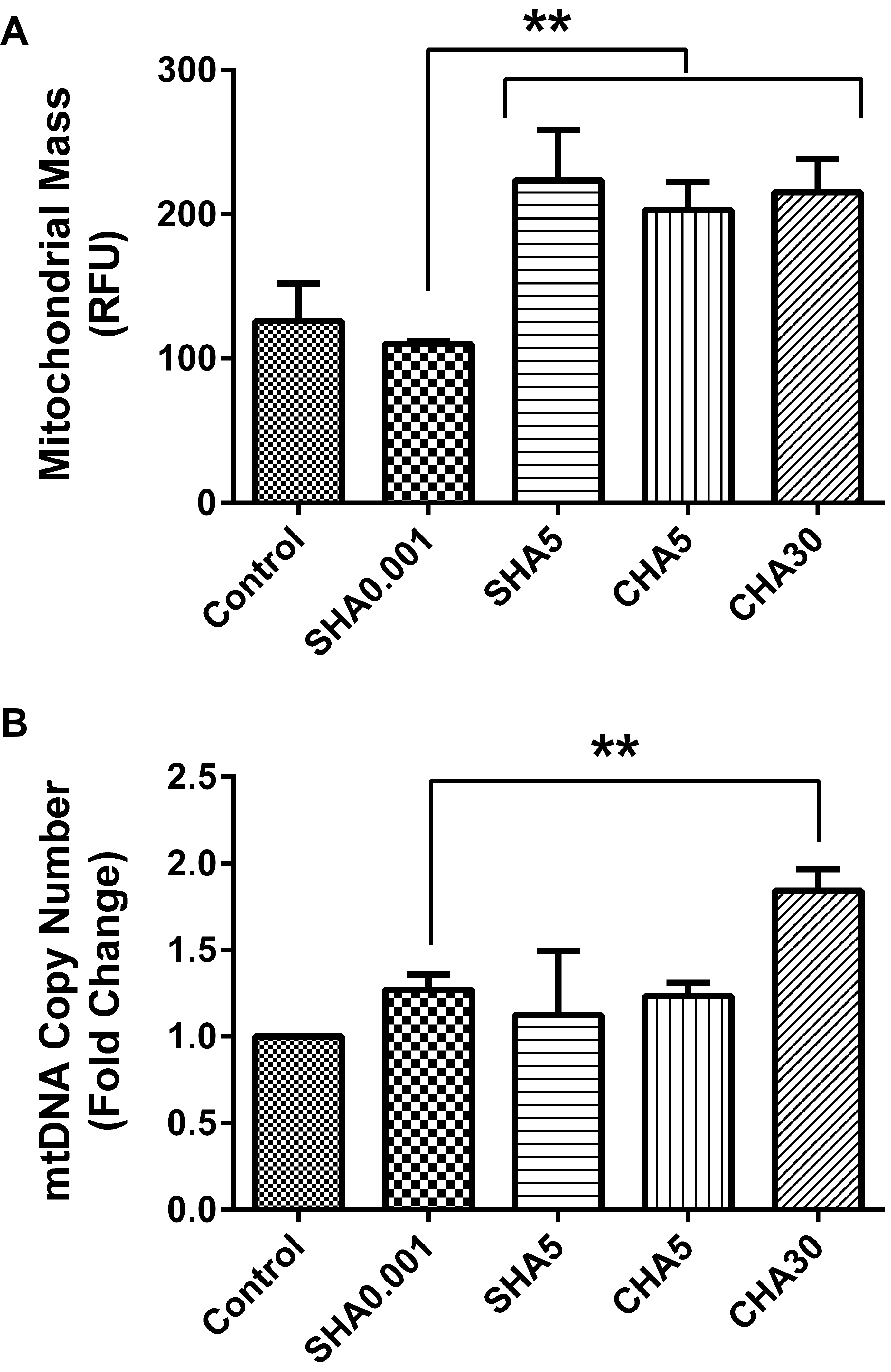 | Fig. 4Hyaluronan-induced fast-proliferative PDMSC had lower mitochondrial biogenesis. (A) Mitochondrial mass; (B) mtDNA copy number; All data is expressed as mean±SD of at least three replicates and analyzed by the paired t-test (*p<0.05, **p<0.01). Mitochondrial mass was analyzed by using PDMSCs transfected with expression plasmid pAS3w-DsRed2-mito. SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, mtDNA: mitochondrial DNA, RFU: relative fluorescence unit. |
Supplementation of hyaluronan in the medium and coated surfaces increases ATP content, modulates lactate production, and attenuates ROS production in PDMSCs
 | Fig. 5Higher ATP content and lactate production in hyaluronan-induced fast-proliferative PDMSCs. (A) ATP content; (B) OCR; (C) lactate production; (D) LDHA levels; and (E) ROS levels of PDMSCs cultured on control surface, SHA (0.001 mg/ml and 5 mg/ml), or CHA (5 and 30 μg/cm2). All data is expressed as mean±SD of at least three replicates and analyzed by paired t-test (*p<0.05, **p<0.01). SHA: medium supplemented hyaluronan, CHA: coated hyaluronan, ATP: adenosine triphosphate, OCR: oxygen consumption rate, ROS: reactive oxygen species, RFU: relative fluorescence unit, H2O2: hydrogen peroxide, O2−: superoxide anion. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download