1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002; 287(10):1308–1320. PMID:
11886323.
2. Sharma S, Elliott P, Whyte G, Jones S, Mahon N, Whipp B, et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am J Cardiol. 2000; 86(2):162–168. PMID:
10913477.

3. Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011; 123(6):668–680. PMID:
21321183.

4. Argulian E, Chaudhry FA. Stress testing in patients with hypertrophic cardiomyopathy. Prog Cardiovasc Dis. 2012; 54(6):477–482. PMID:
22687588.

5. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002; 346(11):793–801. PMID:
11893790.

6. Gulati M, Black HR, Shaw LJ, Arnsdorf MF, Merz CN, Lauer MS, et al. The prognostic value of a nomogram for exercise capacity in women. N Engl J Med. 2005; 353(5):468–475. PMID:
16079370.

7. Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003; 290(12):1600–1607. PMID:
14506119.

8. Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, et al. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long-term outcomes. JACC Cardiovasc Imaging. 2014; 7(1):26–36. PMID:
24290569.
9. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003; 348(4):295–303. PMID:
12540642.

10. Finocchiaro G, Haddad F, Knowles JW, Caleshu C, Pavlovic A, Homburger J, et al. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy: a potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail. 2015; 3(5):408–418. PMID:
25863972.
11. Peteiro J, Bouzas-Mosquera A, Fernandez X, Monserrat L, Pazos P, Estevez-Loureiro R, et al. Prognostic value of exercise echocardiography in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2012; 25(2):182–189. PMID:
22137254.

12. Peteiro J, Fernandez X, Bouzas-Mosquera A, Monserrat L, Méndez C, Rodriguez-Garcia E, et al. Exercise echocardiography and cardiac magnetic resonance imaging to predict outcome in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015; 16(4):423–432. PMID:
25428944.

13. Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail. 2012; 5(4):535–546. PMID:
22811549.
14. Bouabdallaoui N, Ennezat PV, Durand E, Puymirat E, Macron L. Late gadolinium enhancement cardiac magnetic resonance imaging in prognostic assessment of hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013; 14(10):1024. PMID:
23644935.

15. Sorajja P, Allison T, Hayes C, Nishimura RA, Lam CS, Ommen SR. Prognostic utility of metabolic exercise testing in minimally symptomatic patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2012; 109(10):1494–1498. PMID:
22356797.

16. Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014; 35(39):2733–2779. PMID:
25173338.

17. Elliott P. Hypertrophic cardiomyopathy. Circulation. 2018; 138(14):1399–1401. PMID:
30354358.

18. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004; 363(9424):1881–1891. PMID:
15183628.

19. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003; 42(9):1687–1713. PMID:
14607462.

20. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986; 57(6):450–458. PMID:
2936235.

21. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006; 114(21):2232–2239. PMID:
17088454.

22. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011; 58(25):e212–e260. PMID:
22075469.
23. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28(1):1–39.e14. PMID:
25559473.

24. Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M, et al. Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol. 1991; 17(6):1334–1342. PMID:
2016451.

25. Stewart RA, Kittelson J, Kay IP. Statistical methods to improve the precision of the treadmill exercise test. J Am Coll Cardiol. 2000; 36(4):1274–1279. PMID:
11028483.

26. Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA guidelines for exercise testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 1997; 30(1):260–311. PMID:
9207652.
27. Olivotto I, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1999; 33(7):2044–2051. PMID:
10362212.

28. Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation. 1997; 96(9):2987–2991. PMID:
9386166.

29. Kim EK, Lee SC, Hwang JW, Chang SA, Park SJ, On YK, et al. Differences in apical and non-apical types of hypertrophic cardiomyopathy: a prospective analysis of clinical, echocardiographic, and cardiac magnetic resonance findings and outcome from 350 patients. Eur Heart J Cardiovasc Imaging. 2016; 17(6):678–686. PMID:
26245912.

30. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016; 29(4):277–314. PMID:
27037982.

31. Biagini E, Spirito P, Rocchi G, Ferlito M, Rosmini S, Lai F, et al. Prognostic implications of the Doppler restrictive filling pattern in hypertrophic cardiomyopathy. Am J Cardiol. 2009; 104(12):1727–1731. PMID:
19962484.

32. Thaman R, Esteban MT, Barnes S, Gimeno JR, Mist B, Murphy R, et al. Usefulness of N-terminal pro-B-type natriuretic peptide levels to predict exercise capacity in hypertrophic cardiomyopathy. Am J Cardiol. 2006; 98(4):515–519. PMID:
16893708.

33. Tesic M, Seferovic J, Trifunovic D, Djordjevic-Dikic A, Giga V, Jovanovic I, et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. J Cardiol. 2017; 70(4):323–328. PMID:
28336204.

34. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005; 46(3):480–487. PMID:
16053962.

35. Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J. 2017; 38(46):3434–3440. PMID:
29020402.

36. Choi YJ, Kim HK, Lee SC, Park JB, Moon I, Park J, et al. Validation of the hypertrophic cardiomyopathy risk-sudden cardiac death calculator in Asians. Heart. 2019; 105(24):1892–1897. PMID:
31383719.

37. Suzuki K, Akashi YJ. Exercise stress echocardiography in hypertrophic cardiomyopathy. J Echocardiogr. 2017; 15(3):110–117. PMID:
28501918.

38. Sorensen LL, Liang HY, Pinheiro A, Hilser A, Dimaano V, Olsen NT, et al. Safety profile and utility of treadmill exercise in patients with high-gradient hypertrophic cardiomyopathy. Am Heart J. 2017; 184:47–54. PMID:
27892886.

39. Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56(11):875–887. PMID:
20667520.

40. O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56(11):867–874. PMID:
20688032.

41. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014; 130(6):484–495. PMID:
25092278.

42. Hirota Y, Furubayashi K, Kaku K, Shimizu G, Kino M, Kawamura K, et al. Hypertrophic nonobstructive cardiomyopathy: a precise assessment of hemodynamic characteristics and clinical implications. Am J Cardiol. 1982; 50(5):990–997. PMID:
6890306.

43. Bonow RO, Frederick TM, Bacharach SL, Green MV, Goose PW, Maron BJ, et al. Atrial systole and left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. Am J Cardiol. 1983; 51(8):1386–1391. PMID:
6682616.

44. Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog Cardiovasc Dis. 1985; 28(1):1–83. PMID:
3160067.

45. Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987; 10(4):733–742. PMID:
3655141.

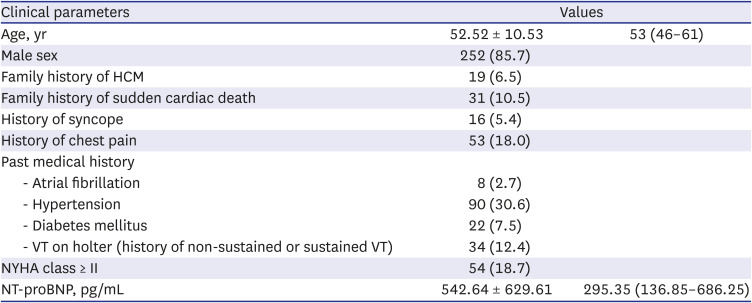
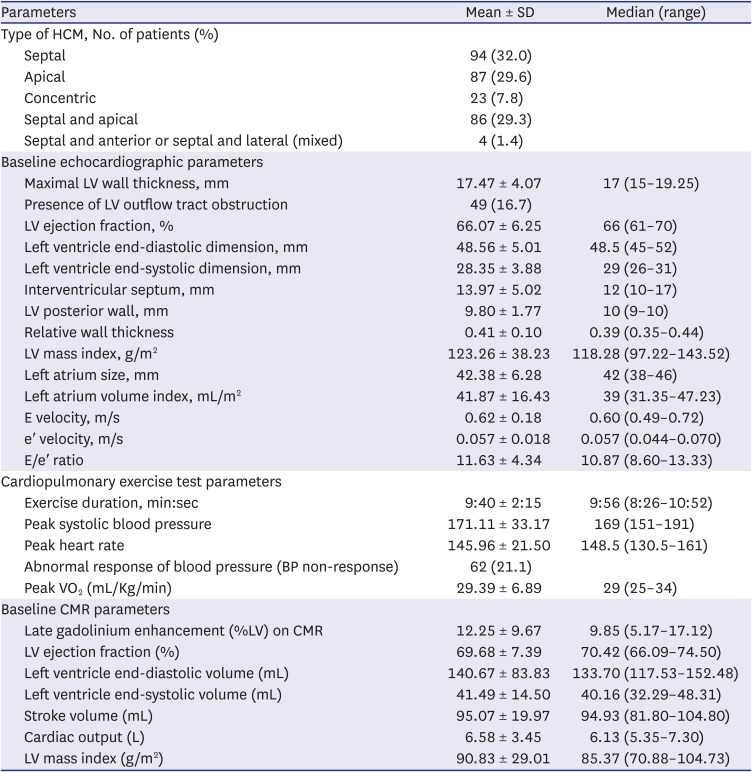
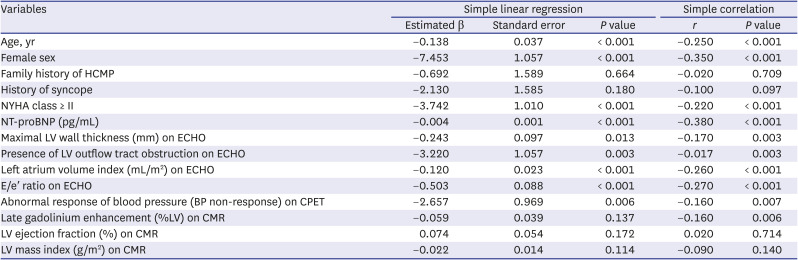
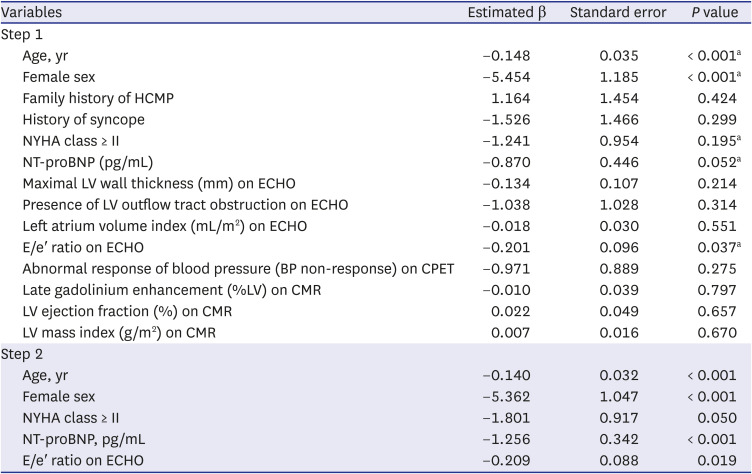




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download