INTRODUCTION
Since the outbreak of coronavirus disease 2019 (COVID-19), the pandemic has lasted over a year and has resulted in more than 100 million people infected. After the first vaccine from Pfizer-BioNTech (BNT162b2) was launched on December 31, 2020, many vaccines were developed including AstraZeneca (AZD1222), Serum Institute of India (Covishield), Sinopharm (severe acute respiratory syndrome-coronavirus-2 [SARS-CoV-2] vaccine), Sinovac (SARS-CoV-2 vaccine), Moderna (mRNA 1273), Johnson & Johnson-Janssen (Ad26.COV 2.S), Gamaleya National Center (Sputnik V), and most recently, BioCubaFarma (Abdala). In addition, 24 vaccines are in use or under evaluation within the WHO EUL/PQ process.
1 Among the vaccines, AZD1222 (Vaxzevria, ChAdOx1-SARS-COV-2, previously AstraZeneca COVID-19 vaccine) is based on a replication-deficient simian adenovirus vector. BNT162b2 (Comirnaty, Pfizer-BioNTech Covid-19 vaccine) is an mRNA vaccine containing instruction for producing a protein from SARS-CoV-2 but does not contain the virus and works by preparing the body to defend itself against COVID 19. Like any vaccine, the above two can cause minor or more serious but rare adverse events including hypersensitivity, anaphylaxis, and autoimmune/inflammatory reactions including Guillain-Barre syndrome (GBS), myocarditis/pericarditis (especially for mRNA vaccines such as Comirnaty), and thrombosis with thrombocytopenia syndrome (especially for adenovirus vector-based vaccines such as Vaxzevria).
GBS is a common immune-mediated acute polyradiculoneuropathy typically occurring after a preceding infection (also termed post-infectious autoimmune polyneuropathy) or after vaccine administration in adults.
2 GBS is characterized by rapidly progressive ascending symmetrical paralysis of the extremities with sensory and reflex changes.
3 Up to August 11, 2021, 23 cases of GBS variant, Miller Fisher syndrome, including total 383 GBS following Vaxzevria vaccination and 42 cases of GBS following Comirnaty injection, were reported from UK public health guidance without detailed clinical course or electrodiagnostic results.
4 Herein we present one GBS case after Vaxzevria vaccination and one GBS case after Comirnaty injection with detailed clinical course, serial electromyographic follow-up, and good prognosis after a comprehensive rehabilitation program.
Go to :

CASE DESCRIPTION
Case 1
A 42-year-old pharmacist presenting with both facial palsy and tetraplegia was transferred to a tertiary university hospital from the local clinic on May 21, 2021. Based on his past medical history, he had received the first dose of AstraZeneca COVID-19 vaccine on April 29, 2021. Two weeks later, he developed left facial palsy and bilateral upper and lower limb weakness. He had no recent respiratory or gastrointestinal infection, including that of C. jejuni. There were no other past medical problem except a previous history of tuberculosis treatment before vaccination. At the onset of symptoms, he was admitted to a nearby inpatient clinic and underwent various examinations conducted, including laboratory tests, brain computed tomography (CT) and CT angiography, cerebrospinal fluid analysis, and electrodiagnostic study. Brain work up and simple laboratory tests did not show any abnormalities. Cerebrospinal fluid analysis revealed albumin-cytologic dissociation without sign of infection (3 white blood cells/μL, 10 red blood cells/μL, and protein 62 mg/dL) and electromyogram results showed an early axonal-type polyneuropathy without F-waves indicative of GBS. He was diagnosed with GBS and received conservative treatment and intravenous immunoglobulin (IVIG) therapy. However, the symptoms progressed, and the patient was transferred to our hospital.
Initially, he had bilateral facial palsy without ophthalmoplegia, quadriparesis (proximal GII, distal GIV) symmetrically, areflexia, and preserved sensation. However, he showed swallowing difficulty with respiratory distress with aspiration pneumonia and was intubated for desaturation, and ventilator care administered. Anti-GM1, anti-GD1, and anti-GQ1 IgG and IgM antibodies were all negative. After the second IVIG with plasma exchange therapy, his weakness and respiratory function began to recover; the patient was transferred to our rehabilitation department and started on comprehensive rehabilitation therapy.
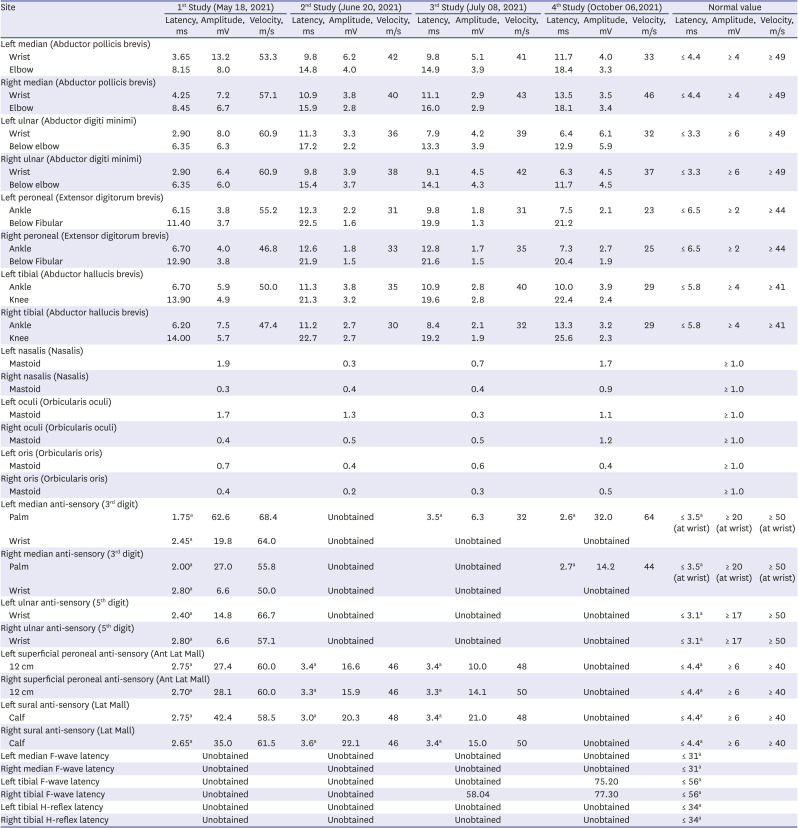
Electrodiagnostic study was performed at 1, 5, 8, and 20 weeks after symptom onset (
Table 1). On the first electromyography (EMG), acute diffuse polyneuropathy with bilateral facial neuropathy was observed without F-waves in all tested nerves and unobtainable blink reflexes. The second EMG showed exacerbated results with more prominent axonal injury and profuse abnormal spontaneous activities on needle examination; recovery signs with F-wave return in the lower limb were observed on the third EMG.
Table 1
Nerve conduction study in case 1
|
Site |
1st Study (May 18, 2021) |
2nd Study (June 20, 2021) |
3rd Study (July 08, 2021) |
4th Study (October 06,2021) |
Normal value |
|
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
|
Left median (Abductor pollicis brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wrist |
3.65 |
13.2 |
53.3 |
9.8 |
6.2 |
42 |
9.8 |
5.1 |
41 |
11.7 |
4.0 |
33 |
≤ 4.4 |
≥ 4 |
≥ 49 |
|
Elbow |
8.15 |
8.0 |
|
14.8 |
4.0 |
|
14.9 |
3.9 |
|
18.4 |
3.3 |
|
|
|
|
|
Right median (Abductor pollicis brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wrist |
4.25 |
7.2 |
57.1 |
10.9 |
3.8 |
40 |
11.1 |
2.9 |
43 |
13.5 |
3.5 |
46 |
≤ 4.4 |
≥ 4 |
≥ 49 |
|
Elbow |
8.45 |
6.7 |
|
15.9 |
2.8 |
|
16.0 |
2.9 |
|
18.1 |
3.4 |
|
|
|
|
|
Left ulnar (Abductor digiti minimi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wrist |
2.90 |
8.0 |
60.9 |
11.3 |
3.3 |
36 |
7.9 |
4.2 |
39 |
6.4 |
6.1 |
32 |
≤ 3.3 |
≥ 6 |
≥ 49 |
|
Below elbow |
6.35 |
6.3 |
|
17.2 |
2.2 |
|
13.3 |
3.9 |
|
12.9 |
5.9 |
|
|
|
|
|
Right ulnar (Abductor digiti minimi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wrist |
2.90 |
6.4 |
60.9 |
9.8 |
3.9 |
38 |
9.1 |
4.5 |
42 |
6.3 |
4.5 |
37 |
≤ 3.3 |
≥ 6 |
≥ 49 |
|
Below elbow |
6.35 |
6.0 |
|
15.4 |
3.7 |
|
14.1 |
4.3 |
|
11.7 |
4.5 |
|
|
|
|
|
Left peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ankle |
6.15 |
3.8 |
55.2 |
12.3 |
2.2 |
31 |
9.8 |
1.8 |
31 |
7.5 |
2.1 |
23 |
≤ 6.5 |
≥ 2 |
≥ 44 |
|
Below Fibular |
11.40 |
3.7 |
|
22.5 |
1.6 |
|
19.9 |
1.3 |
|
21.2 |
|
|
|
|
|
|
Right peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ankle |
6.70 |
4.0 |
46.8 |
12.6 |
1.8 |
33 |
12.8 |
1.7 |
35 |
7.3 |
2.7 |
25 |
≤ 6.5 |
≥ 2 |
≥ 44 |
|
Below Fibular |
12.90 |
3.8 |
|
21.9 |
1.5 |
|
21.6 |
1.5 |
|
20.4 |
1.9 |
|
|
|
|
|
Left tibial (Abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ankle |
6.70 |
5.9 |
50.0 |
11.3 |
3.8 |
35 |
10.9 |
2.8 |
40 |
10.0 |
3.9 |
29 |
≤ 5.8 |
≥ 4 |
≥ 41 |
|
Knee |
13.90 |
4.9 |
|
21.3 |
3.2 |
|
19.6 |
2.8 |
|
22.4 |
2.4 |
|
|
|
|
|
Right tibial (Abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ankle |
6.20 |
7.5 |
47.4 |
11.2 |
2.7 |
30 |
8.4 |
2.1 |
32 |
13.3 |
3.2 |
29 |
≤ 5.8 |
≥ 4 |
≥ 41 |
|
Knee |
14.00 |
5.7 |
|
22.7 |
2.7 |
|
19.2 |
1.9 |
|
25.6 |
2.3 |
|
|
|
|
|
Left nasalis (Nasalis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.9 |
|
|
0.3 |
|
|
0.7 |
|
|
1.7 |
|
|
≥ 1.0 |
|
|
Right nasalis (Nasalis) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
0.3 |
|
|
0.4 |
|
|
0.4 |
|
|
0.9 |
|
|
≥ 1.0 |
|
|
Left oculi (Orbicularis oculi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.7 |
|
|
1.3 |
|
|
0.3 |
|
|
1.1 |
|
|
≥ 1.0 |
|
|
Right oculi (Orbicularis oculi) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
0.4 |
|
|
0.5 |
|
|
0.5 |
|
|
1.2 |
|
|
≥ 1.0 |
|
|
Left oris (Orbicularis oris) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
0.7 |
|
|
0.4 |
|
|
0.6 |
|
|
0.4 |
|
|
≥ 1.0 |
|
|
Right oris (Orbicularis oris) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
0.4 |
|
|
0.2 |
|
|
0.3 |
|
|
0.5 |
|
|
≥ 1.0 |
|
|
Left median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Palm |
1.75a
|
62.6 |
68.4 |
Unobtained |
3.5a
|
6.3 |
32 |
2.6a
|
32.0 |
64 |
≤ 3.5a (at wrist) |
≥ 20 (at wrist) |
≥ 50 (at wrist) |
|
Wrist |
2.45a
|
19.8 |
64.0 |
Unobtained |
Unobtained |
Unobtained |
|
|
|
|
Right median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
|
|
|
|
|
Palm |
2.00a
|
27.0 |
55.8 |
Unobtained |
Unobtained |
2.7a
|
14.2 |
44 |
≤ 3.5a (at wrist) |
≥ 20 (at wrist) |
≥ 50 (at wrist) |
|
Wrist |
2.80a
|
6.6 |
50.0 |
Unobtained |
Unobtained |
Unobtained |
|
|
|
|
Left ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
|
|
|
Wrist |
2.40a
|
14.8 |
66.7 |
Unobtained |
Unobtained |
Unobtained |
≤ 3.1a
|
≥ 17 |
≥ 50 |
|
Right ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
|
|
|
Wrist |
2.80a
|
6.6 |
57.1 |
Unobtained |
Unobtained |
Unobtained |
≤ 3.1a
|
≥ 17 |
≥ 50 |
|
Left superficial peroneal anti-sensory (Ant Lat Mall) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12 cm |
2.75a
|
27.4 |
60.0 |
3.4a
|
16.6 |
46 |
3.4a
|
10.0 |
48 |
Unobtained |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Right superficial peroneal anti-sensory (Ant Lat Mall) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12 cm |
2.70a
|
28.1 |
60.0 |
3.3a
|
15.9 |
46 |
3.3a
|
14.1 |
50 |
Unobtained |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Left sural anti-sensory (Lat Mall) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calf |
2.75a
|
42.4 |
58.5 |
3.0a
|
20.3 |
48 |
3.4a
|
21.0 |
48 |
Unobtained |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Right sural anti-sensory (Lat Mall) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calf |
2.65a
|
35.0 |
61.5 |
3.6a
|
22.1 |
46 |
3.4a
|
15.0 |
50 |
Unobtained |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Left median F-wave latency |
Unobtained |
Unobtained |
Unobtained |
Unobtained |
≤ 31a
|
|
|
|
Right median F-wave latency |
Unobtained |
Unobtained |
Unobtained |
Unobtained |
≤ 31a
|
|
|
|
Left tibial F-wave latency |
Unobtained |
Unobtained |
Unobtained |
75.20 |
≤ 56a
|
|
|
|
Right tibial F-wave latency |
Unobtained |
Unobtained |
58.04 |
77.30 |
≤ 56a
|
|
|
|
Left tibial H-reflex latency |
Unobtained |
Unobtained |
Unobtained |
Unobtained |
≤ 34a
|
|
|
|
Right tibial H-reflex latency |
Unobtained |
Unobtained |
Unobtained |
Unobtained |
≤ 34a
|
|
|

After comprehensive rehabilitation for approximately 4 weeks, contrary to remaining abnormal EMG results, his muscle strength, including respiration and swallowing, recovered very quickly to near normal; he was discharged and could walk without assistance.
5 However, the bilateral facial palsy was managed continuously in a nearby local clinic. One month after discharge, his muscle strength was normalized, and facial weakness was in recovery causing minor discomfort. Second follow up showed much improved facial weakness.
Case 2
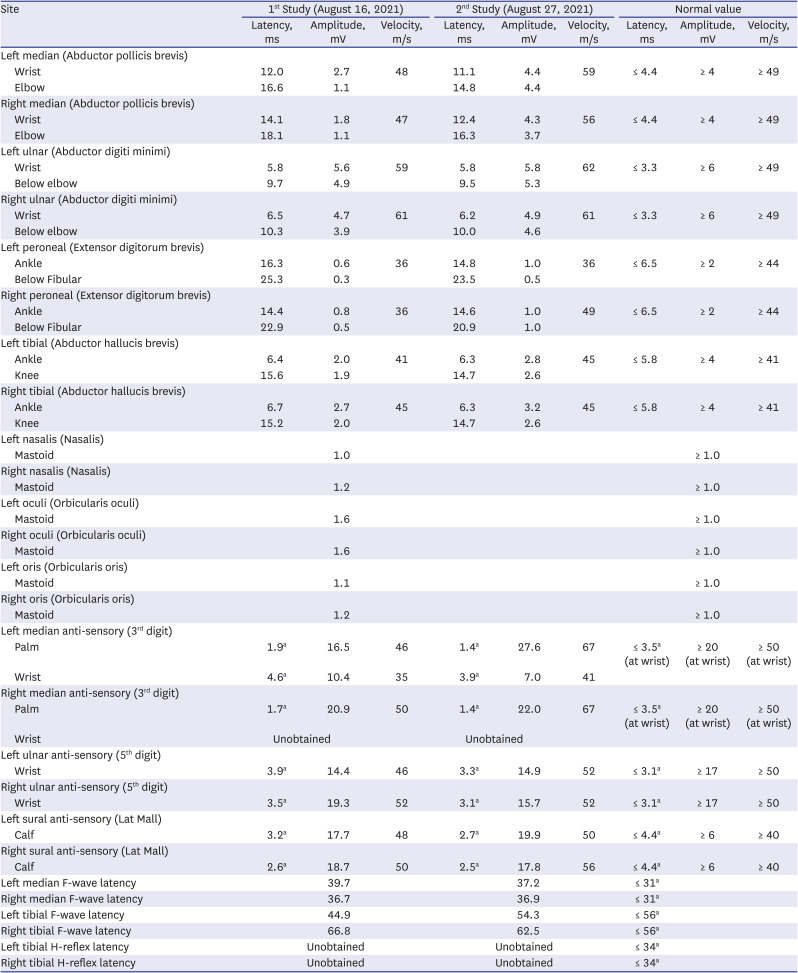
A 48-year-old female complaining of both calf numbness and weakness visited our ER on August 3, 2021, after her first dose of Pfizer COVID-19 vaccine on July 16, 2021. Two weeks later, she developed severe myalgia, numbness with bilateral weakness, and left facial palsy with slight dysarthria. She had underlying hypertension and diabetes without medication. There was no recent history of respiratory or gastrointestinal infection. Severe myalgia presented first, followed by progressive weakness and numbness in all limbs. On the initial examination, she showed paraparesis (G5 in upper, G3 in lower limbs symmetrically) and high blood pressure (200/160 mmHg) without fever. Her brain and spine CT/MR were negative. CSF protein was increased to 150 mg/dL with high albumin (1,025.5 ug/mL) and normal cell (white blood cells 0/μL and 1 red blood cells/μL) and glucose levels. The EMG showed motor-dominant mixed polyneuropathy compatible with GBS. However, anti-GM1, anti-GD1, and anti-GQ1 IgG and IgM antibodies were all negative (
Table 2). There was no oligoclonal band upon examination of CSF. Immediately after admission to the neurologic department, motor weakness progressed to G3 in the upper limbs and G2 in the lower limbs; left facial palsy and dysarthria were aggravated. For these symptoms, IVIG and anti-hypertensive treatment were started immediately. Her weakness began to improve in the toes and fingers with the fifth cycle of IVIG injection. Because she could not sit or eat alone at initial assessment, she was transferred to the rehabilitation department. She showed rapid improvement with comprehensive rehabilitation and improved EMG on serial follow-up.
5 She completely recovered and walked out of the hospital after three weeks of rehabilitation. However, EMG abnormalities, especially in both leg motor conduction, were observed until one week before discharge, indicating discrepancies between current muscle power of the patient and motor nerve conduction state on EMG.
Table 2
Nerve conduction study in case 2
|
Site |
1st Study (August 16, 2021) |
2nd Study (August 27, 2021) |
Normal value |
|
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
Latency, ms |
Amplitude, mV |
Velocity, m/s |
|
Left median (Abductor pollicis brevis) |
|
|
|
|
|
|
|
|
|
|
Wrist |
12.0 |
2.7 |
48 |
11.1 |
4.4 |
59 |
≤ 4.4 |
≥ 4 |
≥ 49 |
|
Elbow |
16.6 |
1.1 |
|
14.8 |
4.4 |
|
|
|
|
|
Right median (Abductor pollicis brevis) |
|
|
|
|
|
|
|
|
|
|
Wrist |
14.1 |
1.8 |
47 |
12.4 |
4.3 |
56 |
≤ 4.4 |
≥ 4 |
≥ 49 |
|
Elbow |
18.1 |
1.1 |
|
16.3 |
3.7 |
|
|
|
|
|
Left ulnar (Abductor digiti minimi) |
|
|
|
|
|
|
|
|
|
|
Wrist |
5.8 |
5.6 |
59 |
5.8 |
5.8 |
62 |
≤ 3.3 |
≥ 6 |
≥ 49 |
|
Below elbow |
9.7 |
4.9 |
|
9.5 |
5.3 |
|
|
|
|
|
Right ulnar (Abductor digiti minimi) |
|
|
|
|
|
|
|
|
|
|
Wrist |
6.5 |
4.7 |
61 |
6.2 |
4.9 |
61 |
≤ 3.3 |
≥ 6 |
≥ 49 |
|
Below elbow |
10.3 |
3.9 |
|
10.0 |
4.6 |
|
|
|
|
|
Left peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
16.3 |
0.6 |
36 |
14.8 |
1.0 |
36 |
≤ 6.5 |
≥ 2 |
≥ 44 |
|
Below Fibular |
25.3 |
0.3 |
|
23.5 |
0.5 |
|
|
|
|
|
Right peroneal (Extensor digitorum brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
14.4 |
0.8 |
36 |
14.6 |
1.0 |
49 |
≤ 6.5 |
≥ 2 |
≥ 44 |
|
Below Fibular |
22.9 |
0.5 |
|
20.9 |
1.0 |
|
|
|
|
|
Left tibial (Abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
6.4 |
2.0 |
41 |
6.3 |
2.8 |
45 |
≤ 5.8 |
≥ 4 |
≥ 41 |
|
Knee |
15.6 |
1.9 |
|
14.7 |
2.6 |
|
|
|
|
|
Right tibial (Abductor hallucis brevis) |
|
|
|
|
|
|
|
|
|
|
Ankle |
6.7 |
2.7 |
45 |
6.3 |
3.2 |
45 |
≤ 5.8 |
≥ 4 |
≥ 41 |
|
Knee |
15.2 |
2.0 |
|
14.7 |
2.6 |
|
|
|
|
|
Left nasalis (Nasalis) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.0 |
|
|
|
|
|
≥ 1.0 |
|
|
Right nasalis (Nasalis) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.2 |
|
|
|
|
|
≥ 1.0 |
|
|
Left oculi (Orbicularis oculi) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.6 |
|
|
|
|
|
≥ 1.0 |
|
|
Right oculi (Orbicularis oculi) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.6 |
|
|
|
|
|
≥ 1.0 |
|
|
Left oris (Orbicularis oris) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.1 |
|
|
|
|
|
≥ 1.0 |
|
|
Right oris (Orbicularis oris) |
|
|
|
|
|
|
|
|
|
|
Mastoid |
|
1.2 |
|
|
|
|
|
≥ 1.0 |
|
|
Left median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
|
|
|
Palm |
1.9a
|
16.5 |
46 |
1.4a
|
27.6 |
67 |
≤ 3.5a (at wrist) |
≥ 20 (at wrist) |
≥ 50 (at wrist) |
|
Wrist |
4.6a
|
10.4 |
35 |
3.9a
|
7.0 |
41 |
|
|
|
|
Right median anti-sensory (3rd digit) |
|
|
|
|
|
|
|
|
|
|
Palm |
1.7a
|
20.9 |
50 |
1.4a
|
22.0 |
67 |
≤ 3.5a (at wrist) |
≥ 20 (at wrist) |
≥ 50 (at wrist) |
|
Wrist |
Unobtained |
Unobtained |
|
|
|
|
Left ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
|
|
|
Wrist |
3.9a
|
14.4 |
46 |
3.3a
|
14.9 |
52 |
≤ 3.1a
|
≥ 17 |
≥ 50 |
|
Right ulnar anti-sensory (5th digit) |
|
|
|
|
|
|
|
|
|
|
Wrist |
3.5a
|
19.3 |
52 |
3.1a
|
15.7 |
52 |
≤ 3.1a
|
≥ 17 |
≥ 50 |
|
Left sural anti-sensory (Lat Mall) |
|
|
|
|
|
|
|
|
|
|
Calf |
3.2a
|
17.7 |
48 |
2.7a
|
19.9 |
50 |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Right sural anti-sensory (Lat Mall) |
|
|
|
|
|
|
|
|
|
|
Calf |
2.6a
|
18.7 |
50 |
2.5a
|
17.8 |
56 |
≤ 4.4a
|
≥ 6 |
≥ 40 |
|
Left median F-wave latency |
39.7 |
37.2 |
≤ 31a
|
|
|
|
Right median F-wave latency |
36.7 |
36.9 |
≤ 31a
|
|
|
|
Left tibial F-wave latency |
44.9 |
54.3 |
≤ 56a
|
|
|
|
Right tibial F-wave latency |
66.8 |
62.5 |
≤ 56a
|
|
|
|
Left tibial H-reflex latency |
Unobtained |
Unobtained |
≤ 34a
|
|
|
|
Right tibial H-reflex latency |
Unobtained |
Unobtained |
≤ 34a
|
|
|

Go to :

DISCUSSION
GBS is an immune-mediated, rapidly progressing polyneuropathy with predominantly motor involvement and often leads to facial and respiratory problems.
6 Although prognosis mostly is favorable, the hospital course varies and demands long rehabilitation in some cases.
7 EMG can play an essential role in diagnosis and monitoring of GBS due to its minimally invasive and repeatable features.
8 In particular, abnormal F-waves on EMG are the hallmark of GBS onset, and the compound muscle action potential (CMAP) amplitude of examined nerves is the best predictor of long-term prognosis.
910 Moreover, GBS can be electrophysiologically divided into various subtypes. Since subtypes of GBS exhibit different mechanisms or antigenic targets, classification of the subtype is critical. There are three existing electrodiagnostic criteria sets.
11 According to Hadden’s criteria, both patients can be classified as primary demyelinating type. Applying criteria of Rajabally and Uncini, both patients are classified as acute inflammatory demyelinating polyneuropathy.
In our first case, the patient showed significant axonal loss and demyelination with abnormal spontaneous activities at both upper and lower limbs on EMG approximately one month after AstraZeneca COVID-19 vaccine; his weakness progressed to the whole body, affecting the proximal and distal parts of all four limbs. The patient’s strength returned to near normal approximately two months after onset. However, his facial palsy remained and abnormal facial EMG was observed after two months. During the second NCS study, the patient showed severe quadriparesis and paresthesia of limbs. All of the upper limb NCSs and lower limb motor NCSs showed severe axonal loss and conduction block. Bilateral median and tibial F-waves were absent. On the third NCS study, improvements were found at both upper and lower motor NCSs. Right tibial F-wave was obtained with delayed latency. His weakness quickly returned to near normal with persistent mild numbness and paresthesia. By the time of the last study, the patient showed no sign of weakness and numbness. Signs of recovery were observed on the fourth NCS study, with improved motor and sensory NCSs, indicating remyelination. Moreover, the left tibial F-wave was obtained, contrary to previous studies. However, sural and superficial peroneal SNAP were unobtained. Previous studies have shown no sign of abnormalities. The patient did not show sensory-related symptoms. To explain the abrupt loss at sural and superficial peroneal SNAP, we could consider reversible conduction failure (RCF),
11 which is common in GBS patients. Although RCF is more common in GBS with axonal loss type, RCF can be observed in GBS with acute inflammatory demyelinating polyneuropathy.
In the second case, the GBS after Pfizer COVID-19 vaccine showed faster recovery then the first case with AstraZeneca COVID-19 vaccine. The symptoms at initial onset after two weeks of vaccination were similar in the cases, with progressive generalized weakness, myalgia, and facial palsy. However, the clinical course was different. Her weakness was milder at the time of nadir, and recovery was faster and complete with one month of total clinical course, and only EMG abnormalities remained, without clinical signs or symptoms of weakness. On the first NCS study, both upper and lower motor NCSs showed decreased amplitude and conduction velocity along with delayed latency. Both lower limb muscles showed greater axonal degeneration and conduction block than the upper limbs, which can explain the more prominent weakness of her lower limbs. The second NCS study showed recovery of both upper and lower limbs. Median and ulnar CMAP amplitude and conduction velocity improved to near normal limits, indicating remyelination. Peroneal and tibial CMAP did not show much improvement in the upper limbs, and significant axonal degeneration and conduction block remained. The patient showed only a mild gait disturbance without weakness and other difficulties.
In other reported cases of GBS in patients vaccinated with AstraZeneca adenovirus vector COVID-19 vaccine, various symptoms were observed.
121314 Among 15 patients, there were nine cases of quadriparesis, 12 of bilateral facial palsy, two of ophthalmoplegia, five of bulbar palsy, nine of sensory impairment, five of limb numbness, and six of respiratory failure.
15 Symptom onset from vaccination occurred in 2 patients within 1 week, 11 patients within 2 weeks, and 2 patients within 4 weeks. In our first case, symptoms began 2 weeks after vaccination; the patient was nearly quadriplegic and experienced bilateral facial palsy and respiratory failure without sensory symptoms at nadir.
Among the 15 reported AstraZeneca vaccine-related GBS patients, 14 electrodiagnostic studies were performed without definite results of needle EMG, and follow-up electrodiagnostic studies were not reported.
4 The NCS results showed 2 patients with axonal loss, 8 with demyelination, and 4 with normal results. In our case of AstraZeneca vaccine-related GBS, serial EMG follow-up showed demyelinating lesion at the initial phase, followed by significant axonal loss and active denervation of the involved muscles. The EMG results finally improved, but the patient showed slightly delayed clinical improvement.
Waheed et al.
16 reported an 82-year-old female who developed GBS after the first dose of Pfizer vaccination and showed a similar clinical course to our second case. The patient recovered without respiratory failure contrary to our first GBS case after the Pfizer COVID-19 vaccine. Another case of GBS developing after the second dose of the Pfizer vaccine was reported in an 82-year-old Italian female.
17
In our cases, different side effects were observed between mRNA vaccine and vaccine delivered using adenovirus vector; the mRNA vaccine showed less severe GBS side effects than the adenovirus vector vaccine.
18 As of August 2021, there have been 383 reports of GBS in the UK following AstraZeneca vaccine and 42 reports following Pfizer vaccine.
4 In the USA, as of July 2021, there have been 100 reports of GBS following the Johnson & Johnson vaccine, also based on replication-deficient simian adenovirus vector.
19 No similar incidence has been identified with the Pfizer vaccine. The World Health Organization has also reviewed the data, noting no increased reports of GBS following mRNA vaccines.
4 Although current reports and evidence suggest increased risk of GBS following AstraZeneca vaccine, data are insufficient to assume a direct relationship.
20 Since the overall incidence of GBS following the AstraZeneca vaccine was greater than that following the Pfizer vaccine, additional caution about GBS is needed for administration of the AstraZeneca vaccine.
We reported two cases of COVID-19 vaccine-related GBS (AstraZeneca adenovirus vector and Pfizer mRNA vaccines). Due to increasing pan-vaccination for COVID-19, clinicians should be aware of this serious neurological complication and any other adverse reactions associated with COVID-19 vaccination to ensure prompt management based on vaccine type.
Ethics statement
We obtained informed consents for publication from the patient.
Go to :




