This article has been
cited by other articles in ScienceCentral.
Abstract
Acute transverse myelitis (ATM) has been reported as rare complication of vaccination. Herein, we report 2 cases of ATM after the administration of an mRNA vaccine for coronavirus disease 2019 (COVID-19). The first one is an 81-year-old man who received the BNT162b2 vaccine. He presented with bilateral hand weakness. Spine magnetic resonance imaging (MRI) showed high signal intensity from the C1 to C3 vertebrae. The second is a 23-year-old woman who received the BNT162b2 vaccine and experienced tingling in her legs. Spine MRI showed a high signal intensity lesion at the conus medullaris. These patients were treated with intravenous methylprednisolone and their symptoms improved slightly. Careful follow-up is needed to identify adverse events after the administration of mRNA vaccines for COVID-19.
Go to :

Graphical Abstract
Go to :

Keywords: Transverse Myelitis, COVID-19 Vaccine, mRNA Vaccine, CNS Demyelinating Disease
INTRODUCTION
The messenger ribonucleic acid (mRNA) vaccine for coronavirus disease 2019 (COVID-19) is a new platform of vaccine. It uses a lipid nanoparticle with RNA modified at the nucleoside-level to code the whole sequence of the spike antigen for the severe acute respiratory syndrome coronavirus-2. This distinguishes it from the adenovirus vector vaccine.
1 The mRNA COVID-19 vaccines are manufactured by Pfizer/BioNTech and Moderna. Both vaccines share the same technology and mechanism of action. The mRNA vaccines have a reported efficacy of up to 95% and are currently used in several countries.
2 In Korea, the initial phase of COVID-19 vaccination using the BNT162b2 vaccine (manufactured by Pfizer) began in February 2021.
Demyelinating diseases of the central nervous system (CNS), such as acute transverse myelitis (ATM), have been reported as rare complications of vaccination.
3 Since COVID-19 vaccination began, there have been several reports of ATM following vaccination using an adenovirus vector vaccine (ChAdOx1nCoV-19, manufactured by AstraZeneca). However, no cases of ATM related to mRNA vaccines have been reported in clinical trials.
45 Here, we report 2 cases of ATM after administration of the BNT162b2 mRNA vaccine.
Go to :

CASE DESCRIPTION
Case 1
An 81-year-old Korean man received the BNT162b2 vaccine on April 29 and May 19, 2021. Three days after the second dose of the vaccine, he presented with bilateral hand weakness and numbness in his fingers. His symptoms progressed over the next 2 weeks. The patient had a history of hypertension and diabetes mellitus, but no history of sensory symptoms. On neurologic examination, Medical Research Council (MRC) grade 2 weakness with paresthesia was observed in both hands and fingers; deep tendon reflexes were exaggerated in the upper limbs.
Spine magnetic resonance imaging (MRI) showed high signal intensity and multifocal nodular enhancement with an ill-defined signal increase on T2-weighted images from the C1 to C3 vertebrae (
Fig. 1A). Brain MRI revealed mild brain atrophy. Cerebrospinal fluid (CSF) examination results were normal (white blood cell [WBC] count, 0 cells/μL; protein level, 28.6 mg/dL). Chest and abdomen computed tomography (CT) revealed no evidence of malignancy. Laboratory tests did not reveal any abnormalities. Clinically relevant antibodies, such as anti-aquaporin 4 antibody, anti-myelin-oligodendrocyte antibody, vasculitis antibodies, and paraneoplastic antibodies, were not detected. The patient was treated with intravenous methylprednisolone (1 g/day for 5 days). This was tapered for 2 weeks using oral prednisolone. The hand weakness improved, however, he continues to experience a limitation of his finger movements after 1 month.
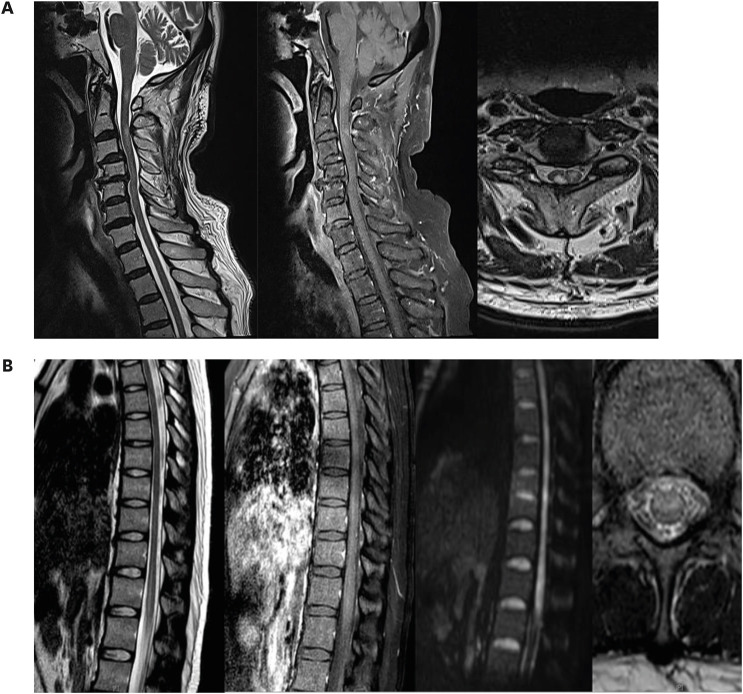 | Fig. 1
(A) Spine MRI showing high signal intensity and multifocal nodular enhancement with an ill-defined signal increase on T2-weighted images from the C1 to C3 vertebrae. (B) Spine MRI showing a high signal intensity lesion without contrast enhancement at the anterior portion of the conus medullaris on T2-weighted images.
MRI = magnetic resonance imaging.

|
Case 2
A 23-year-old Korean woman with no relevant medical history received the BNT162b2 vaccine on August 18, 2021. Three weeks after the first dose of the vaccine, the patient suddenly experienced a tingling sensation in both thighs. Weakness of both legs developed after 1 hour and rapidly progressed. When the patient visited the emergency department 1 day after the onset; she was unable to walk and had urinary retention. On neurologic examination, severe weakness (MRC grade 2) was observed in both legs. The patient no longer experienced a tingling sensation, and sensory examination was normal in both legs. Deep tendon reflexes were absent in both legs. The weakness of her legs further progressed to MRC grade 1 during the first 2 days after hospital admission.
Spine MRI detected a lesion with high signal intensity without contrast enhancement at the anterior portion of the conus medullaris on T2-weighted images (
Fig. 1B). Cortical motor evoked potential (MEP) was absent and the central conduction time of somatosensory evoked potentials (SEP) was prolonged in the lower limbs. Central conduction time was normal for the MEP and SEP of the upper limbs. No abnormal lesions were observed on brain MRI. CSF examination showed normal WBC count (2 cells/μL) and protein levels (33.5 mg/dL). CT of the aorta revealed no evidence of aortic dissection. Results of anti-aquaporin 4, anti-myelin-oligodendrocyte, and anti-neutrophil cytoplasmic antibodies were negative. No evidence of a hypercoagulable state was observed in laboratory tests. The patient was treated with intravenous methylprednisolone (1 g/day for 5 days), which was tapered for 2 months using oral prednisolone. After 3 months, weakness of both legs improved to MRC grade 4. The patient was able to walk with unilateral assistance.
Ethics statement
Informed consent for publication of clinical data was obtained from the case patient.
Go to :

DISCUSSION
In Korea, 2 types of vaccines are available for COVID-19 prophylaxis: viral vector vaccines (manufactured by AstraZeneca and Johnson & Johnson) and mRNA vaccines (Pfizer/BioNTech, Moderna).
2 The safety and detailed side-effect profiles of COVID-19 vaccines are continuously being evaluated. In a study that included healthcare workers who received the BNT162b2 vaccine, approximately 60% of vaccine recipients reported malaise or fatigue and 45% reported myalgia.
6 Cases of myelitis after administration of the BNT162b2 vaccine were rarely reported, and this paper includes the first report of cases of ATM after BNT162b2 vaccination in Asia.
Cases of post-vaccination ATM have been frequently reported following vaccination against hepatitis B virus, diphtheria and tetanus, and influenza.
3 Three cases of serious ATM have been reported following the administration of viral-vector vaccines for COVID-19.
45 However, the pathogenesis of vaccine-associated myelitis remains unclear. It is assumed that recombinant or live attenuated viruses used for vaccination can induce autoimmunity, molecular mimicry, or have epitopes and spreading characteristics similar to those of infectious agents.
7 Although mRNA vaccines do not use recombinant or live attenuated viruses, autoimmune reactions may occur between the SARS-CoV-2 spike protein antibody and tissue proteins or ACE2 receptors.
8 The possibility of spinal cord infarction due to thrombus formation induced by COVID-19 vaccination also cannot be excluded; as there are several reports of thrombosis after COVID-19 vaccination, primarily with the viral vector vaccines.
910
A case series of CNS demyelinating disease after an mRNA vaccine was recently published. It included 1 case of a Caucasian man diagnosed with ATM without multiple sclerosis.
11 While a low incidence of ATM following the administration of mRNA vaccines is expected, a few cases were previously reviewed.
1213
As of March 2, 2021, 51,755,447 doses of various vaccines have been administered in the United States. A total of 9,442 adverse reactions to the vaccines, including 9 cases of ATM, have been reported in the vaccine adverse event reporting system.
12 In Korea, as of August 2021, 13,868,460 residents have been vaccinated. Adverse events have been reported in 28% of the recipients. Out of these adverse events, 4.3% were reported as serious events, excluding death or anaphylaxis.
141516 The vaccines for COVID-19 received emergency approval. There was limited time to observe the side effects after vaccination. Therefore, studies with a longer study period, including observational studies, are needed to elucidate the causal relationship between neurological complications and mRNA vaccination.
Herein, we summarize the first reported cases of ATM after the administration of an mRNA vaccine for COVID-19 prophylaxis in Asian. Careful follow-up is needed to identify adverse events, such as ATM, after the administration of mRNA vaccines for COVID-19.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download