Abstract
Purpose
Methods
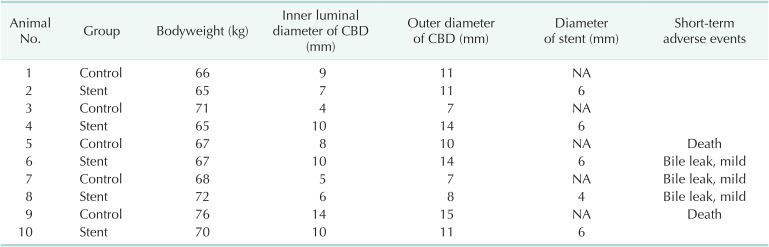
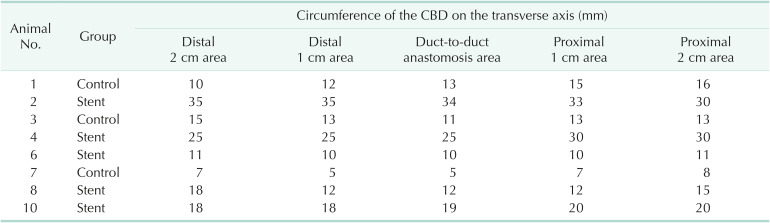
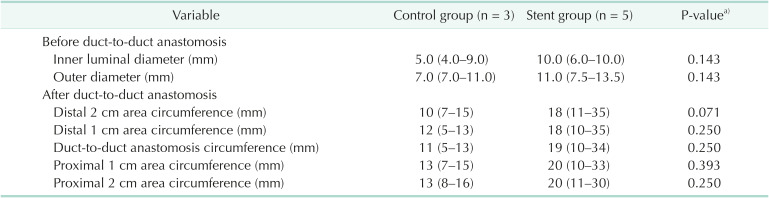
Results
Notes
Fund/Grant Support: This work was supported by grants of 1) The Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (202011B31), 2) The Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (HI20C1488), and 3) The Technology Development Program (S2648083) funded by the Ministry of SMEs and Startups (MSS, Korea), the T2B Infrastructure Center for Digestive Disorders (HI15C0989) funded by the Korean Health Industry Development Institute.
References
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1
Fig. 1
Shape of the biodegradable filaments and stents. (A, B) Scanning electron microscopic images of polydioxanone/magnesium (PDO/Mg) sheath-core monofilaments (A, surface [×50]; B, cross-section [×180]). (C) Prototype biodegradable stents were made using the cross-and-hook knitting handmade method.
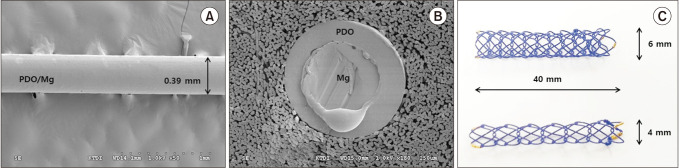
Fig. 2
Trimming of the common bile duct (CBD) in the swine. (A) Exposure and isolation of the CBD. (B) Transverse cutting of the CBD. (C) Measuring the inner diameter of the transected CBD using calipers. (D) Measuring the outer diameter of the transected CBD using calipers.
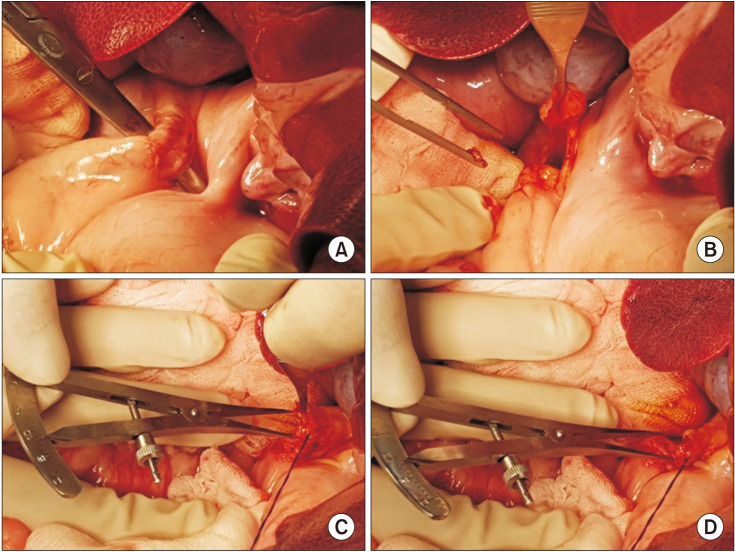
Fig. 3
Process of duct-to-duct anastomosis in the swine. (A, B) Control group. Duct-to-duct anastomosis with posterior wall continuous sutures and anterior wall interrupted suture using 6-0 polypropylene monofilaments (Prolene; Ethicone, Inc., Somerville, NJ, USA) without biodegradable stent (BS) insertion. (C, D) Stent group. After posterior wall continuous suture, a 4-mm or 6-mm polydioxanone/magnesium sheath-core BS was inserted into the transected CBD. Then, the anterior wall was closed by interrupted sutures.

Fig. 4
Process of cholecystectomy in the swine. (A) Detachment of the gallbladder from the liver bed. (B) Removal of the gallbladder dividing the cystic duct. (C) Gross appearance of cholecystectomy specimen.

Fig. 5
Autopsy findings. (A) Distended intestine due to major bleeding. (B) Dark red mucosal changes in the large intestine due to ischemic changes.
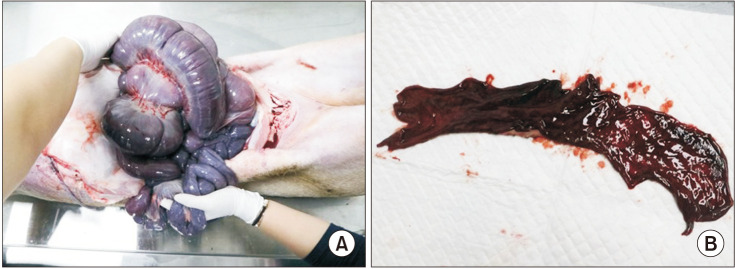
Fig. 6
Fluoroscopic tracking images of the inserted biodegradable stents. The biodegradable stents were observed without deformity until 4 weeks in all swine. However, all biodegradable stents are deformed or disappeared at 6 weeks. The length of the red ladder marks represents the length of the gold marks attached to both ends of the stents without deformity. The red arrow represents the gold marks that partially existed due to the degradation of the stents at 6 weeks.
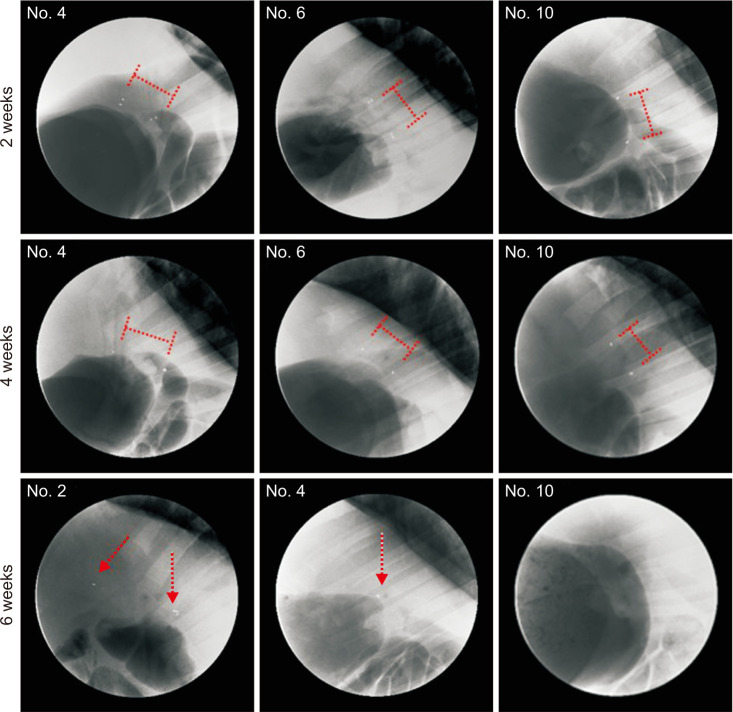
Fig. 7
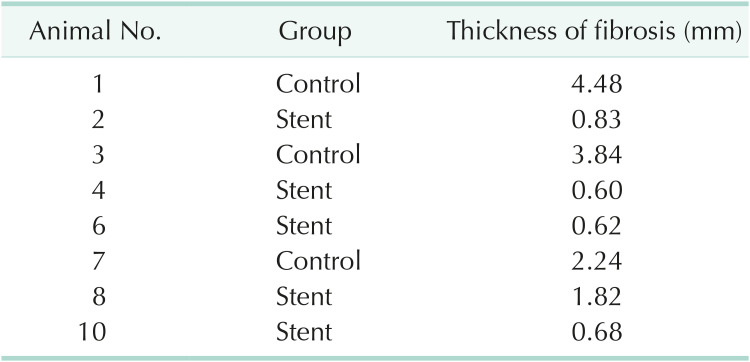
Histopathologic images. (A) Gross images of the common bile ducts. The circumferences of the duct-to-duct anastomosis area (red arrows) in the stent group (specimens 2, 4, 6, 8, and 10) are wider than those in the control group (specimens 1, 3, and 7). (B) Microscopic images of the duct-to-duct anastomosis area. Masson’s trichrome staining of paraffin-embedded tissue revealed much thicker fibrosis (blue color in collagen accumulation) in the control group than in the stent group (×40).





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download