Abstract
Purpose
This study was performed To investigate the use of hydrophilic guidewires for facilitating catheter advancement during varicose vein treatment using radiofrequency ablation (RFA) or cyanoacrylate closure (CAC).
Methods
From March 2016 to April 2019, 463 limbs of 285 with incompetent great saphenous veins were subjected to RFA (321 limbs of 197 patients) or CAC (142 limbs of 88 patients). Procedure records were reviewed for the use of a hydrophilic guidewire, reason for the guidewire usage, and diameter of the guidewire.
Results
A hydrophilic guidewire was used to facilitate catheter advancement to treat 92 of 463 limbs (19.9%). For RFA, a guidewire was used to treat 53 of 321 limbs (16.5%). Among them, 15 limbs (28.3%) had vasospasm, and 38 limbs (71.7%) had venous tortuosity. For CAC, guidewire was used for 39 of 142 limbs (27.5%). Among them, 10 limbs (25.6%) had vasospasm, 23 limbs (59.0%) had venous tortuosity, and 6 limbs (15.4%) had repeated engagement of a J-tip guidewire into the varicose tributaries. In CAC, the frequency of hydrophilic guidewire usage was higher than that in RFA (P = 0.006). All varicose vein treatment sessions were technically successful.
Go to : 
Varicose vein is a common disease. In Western countries, approximately 23% of adults have varicose veins, and 6% of adults suffer from more advanced forms of chronic venous disease, including skin changes and healed or active venous ulcers [1].
In the last decade, various treatment options for varicose veins have emerged. Contrary to conventional surgical treatments, such as high ligation and stripping, less-invasive treatment options have been introduced. Ranging from endovenous laser ablation, radiofrequency ablation (RFA), cyanoacrylate closure (CAC), and mechanochemical ablation, these endovenous treatment options demonstrate an occlusion rate comparable to that of surgical treatment [23]. Simultaneously, these endovenous techniques have several advantages over surgical treatment owing to their minimally invasive nature, requirement of only local anesthesia, shorter hospital stay, faster recovery, earlier ambulation, and lower periprocedural morbidity [4].
Hydrophilic-coated guidewires were introduced in the early 1990s. They have a polymer coating, minimizing friction within the lesions. Therefore, many operators prefer these wires for navigating the tortuous anatomy and crossing tight lesions [5]. The performance of several hydrophilic-coated guidewires has been individually described in the clinical practice [67891011121314]. Kähler et al. [6] reported that the hydrophilic guidewire is an effective tool to treat chronic coronary occlusions, even when recanalization attempts with conventional guidewires fail. Moreover, Poncyliusz et al. [7] reported that the use of hydrophilic guidewires has increased the technical success rate of peripheral percutaneous transluminal angioplasty, particularly in occlusion and more complicated lesions.
When performing endovenous treatment of the varicose vein using RFA or CAC, these catheters are structured to allow hydrophilic guidewires to pass through. We decided to see if this hydrophilic guidewire could change the technical success rate. Therefore, the objective of this study was to investigate the effectiveness of hydrophilic guidewire usage in facilitating catheter advancement during varicose vein treatment using RFA and CAC.
Go to : 
This is a retrospective study conducted at Konkuk University Medical Center with Institutional Review Board approval (No. 2020-08-012). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature. From March 2016 to April 2019, 285 patients diagnosed with varicose veins in unilateral or bilateral legs as well as incompetence in great saphenous vein (GSV) were included in this study. Duplex ultrasound (US) using an EPIQ 7 (Philips, Bothell, WA, USA) with an eL18-4 MHz linear transducer was performed in the standing position and a neutral state with compression and release maneuver of distal venous segments to determine refluxes into the GSV. Reflux was defined as reverse flow in the GSV for more than 0.5 seconds after releasing calf or thigh compression.
Patients who were less than 18 years old and those with deep venous obstruction, deep vein thrombosis, superficial thrombophlebitis, congenital vascular malformation, nonpalpable pedal pulses associated with peripheral arterial disease, inability to ambulate, poor general health condition, pregnancy, nursing, or planning a pregnancy at some time during the course of treatment were excluded.
In our institute, RFA was recommended when a patient had 3 or more saphenous veins to be treated in 1 session, and when GSV was traveling close to the skin along with the extrafascial space at the thigh level. If the patient was too afraid of the pain associated with the procedure or had discomfort in wearing compression stockings after the procedure, CAC was recommended. However, basically, the patients could choose the type of procedure they would receive after discussing the pros and cons.
Before treatment for GSV insufficiency, all lesions were classified based on the clinical, etiological, anatomical, and pathophysiological classification and assigned a venous clinical severity score (VCSS).
After obtaining written informed consent, the patient was brought to the procedure room, placed in a supine position, and draped in the usual sterile fashion from the groin to the ankle. Two practitioners (S.W.P. and J.H.H.) performed all procedures independently.
RFA was performed under conscious sedation. At the start of sedation, dexmedetomidine (200 µg/50 mL; Precedex Premix injection, Pfizer Pharmaceuticals Korea Ltd., Seoul, Korea) was administered intravenously as a loading dose of 1 µg/kg/hour over 15 minutes, which was followed by a maintenance dose of 0.2 µg/kg/hour throughout the procedure using an infusion pump (syringe pump TE-331, Terumo, Tokyo, Japan). If sufficient sedation had not been achieved after 15 minutes of induction of sedation, midazolam (Bukwang Pharmaceutical, Co., Ltd., Seoul, Korea) bolus was administered repeatedly in increments of 1 mg until sedation.
The GSV was punctured using a 21-gauge micropuncture needle under US guidance. For the RFA procedure, the GSV was punctured at the knee joint level. For the CAC procedure, the GSV was punctured at the calf level. A puncture was made at a higher level for the RFA, in order to avoid thermal damage to the GSV.
After successfully puncturing the GSV, a 0.018-inch wire and 4-Fr or 5-Fr micro sheath (Cook Medical, Bloomington, IN, USA) were introduced into the GSV. A 0.035-inch guidewire was then exchanged, allowing the insertion of a 7-Fr sheath (Terumo).
During the RFA procedure using the ClosureFast system (Medtronic Vascular Inc., Santa Rosa, CA, USA), the device was advanced through the sheath up to the SFJ without guidewire assistance. US guidance was applied throughout to ensure that the RF catheter was properly advanced along the GSV without engaging the other branch vessels. When the RF catheter advancement was troublesome, a 0.025-inch hydrophilic guidewire (Radiofocus Guide Wire M, angled tip shape; Terumo) was used under US guidance to facilitate catheter advancement in accordance with the instructions for use. We attempted to line up the distal part (around the tip) of the hydrophilic guidewire and troublesome part of the GSV on the same longitudinal plane of US view as accurately as possible and then repeated the process of carefully advancing the guidewire while rotating it to make it pass through the problematic segment. In cases where successful passage of the guidewire in the longitudinal plane could not be confirmed, the transverse plane was applied alternately. If the 0.025-inch guidewire failed to advance through the RF catheter, a 0.018-inch hydrophilic guidewire (Radiofocus Guide Wire M, angled tip shape; Terumo) was used instead. After verifying the catheter tip position 2 cm below the saphenofemoral junction (SFJ), perivenous tumescent anesthesia was induced under US guidance with the 0.05% lidocaine solution using a 25-gauge needle along the course of GSV. RFA was achieved using an intraluminal RF catheter (7-cm heating element), with segmental energy delivered at 120℃ in 20-second cycles. Two cycles of ablation were performed for the initial venous segment from the proximal GSV, 2 cm below the SFJ, followed by 1 cycle ablation per segment for the remaining GSV. Following GSV ablation, remaining varicosities were subjected to localized treatment, such as ambulatory phlebectomy or sclerotherapy.
During the CAC procedure using the VenaSeal closure system (Medtronic Vascular Inc.), a 0.035-inch J-wire guidewire was introduced into the vein. If passage of the J-wire guidewire was not achieved, a 0.035-inch hydrophilic guidewire (Radiofocus angled type; Terumo) was used to advance into the SFJ. A 5-Fr delivery catheter in a 7-Fr introducer was advanced into the SFJ and positioned 5 cm caudal to the SFJ. With compression of the GSV by a US probe 2 cm proximal to the delivery catheter tip, double injections of cyanoacrylate were administered 1 cm apart at this location, followed by a 3-cm pullback and 3-minute localized manual compression over the injected venous segment. Subsequently, a single injection and 30-second compression was repeated at every 3-cm interval for closure of the remaining GSV. The sheath and catheter were removed, and manual compression was applied to the puncture site until hemostasis was achieved.
Technical success of the RFA or CAC procedure was defined as successful access and traversal of the segment planned for ablation or closure and delivery of thermal energy or cyanoacrylate to the incompetent GSV. Patients were evaluated using duplex US clinically after 1 week and 1, 3, 6, and 12 months. To define successful treatment, no compressibility of the treated veins and no blood flow within the ablated or closed GSV were determined using duplex US. The frequency of usage of the hydrophilic guidewire, reason for usage of the hydrophilic guidewire, and diameter of the hydrophilic guidewire were recorded. The reasons for usage of the hydrophilic guidewire were divided into tortuosity, vasospasm, and repeated engagement of a J-tip guidewire into the varicose tributaries. Tortuosity refers to when a saphenous venous segment runs in a curved course on US where the radiofrequency (RF) catheter or J-tip guidewire does not advance any further. Vasospasm refers to a case in which the diameter of the saphenous vein is markedly decreased compared to the diameter before puncture, and the RF catheter or J-tip guidewire does not advance further in this segment. Repeated engagement of a J-tip guidewire is when the RF catheter or J-tip guidewire repeatedly enters the branch of the varicose vein connected to the saphenous vein. In addition, the VCSS and complications, including pain, bruise, paresthesia, and deep vein thrombosis, were recorded during the follow-up period.
Data from the assessment were recorded, and analyses were performed using PASW Statistics ver. 18.0 (IBM Corp., Armonk, NY, USA). Values are expressed as mean ± standard deviation or number and percentage (n, %). All comparisons of numerical variables between groups were made by using Mann-Whitney U-test. The comparisons between groups were performed with chi-square and Fisher exact tests for categorical variables. The Kaplan-Meier survival curve analysis was used to calculate the occlusion rates.
Go to : 
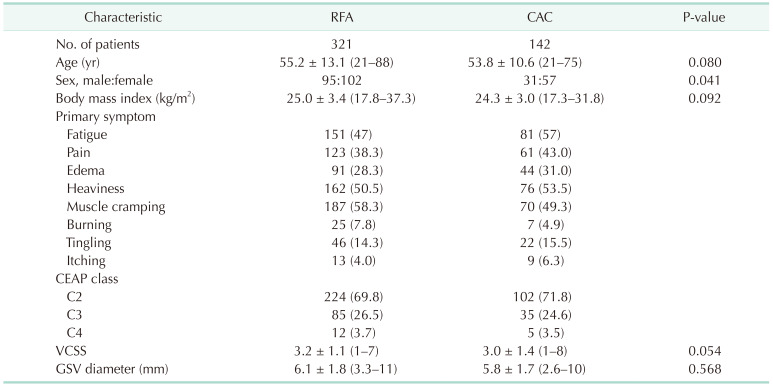
A total of 463 limbs of 285 patients (male, 126 and female, 159; mean age, 54.8 years; age range, 21–88 years) underwent treatment for GSV insufficiency in the angio-suite. Among them, RFA was performed for 321 limbs of 197 patients, and CAC was performed for 142 limbs of 88 patients. Table 1 summarizes the demographic and baseline data of patients.
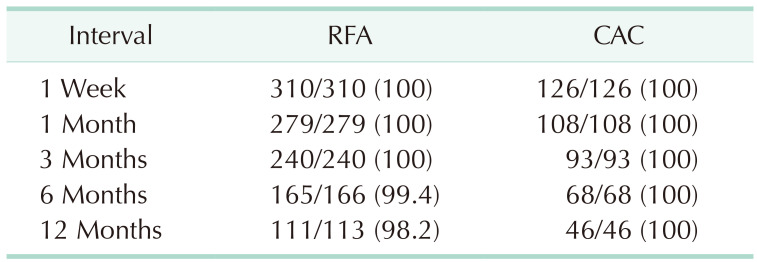
A hydrophilic guidewire was used in 19.9% of cases (92 of 463 limbs) to facilitate catheter advancement during RFA and CAC. Regarding RFA, 1-week follow-up results were obtained for 191 patients and 310 limbs. Complete closure of the GSV was seen for all 310 limbs after 1 week (100%), 279 of 279 limbs after 1 month (100%), 240 of 240 limbs after 3 months (100%), 165 of 166 limbs after 6 months (99.4%), and 111 of 113 limbs after 1 year (98.2%). Two GSVs were recanalized with the recurrence of reflux during follow-up, one at 6 months, and the other one at 1 year, and the hydrophilic guidewire had been used for none of these limbs. Regarding CAC, 1-week follow-up results were obtained for 88 patients and 142 limbs. Complete closure of the GSV was seen for all 142 limbs (100%) after 1 week, 108 of 108 limbs after 1 month (100%), 93 of 93 limbs after 3 months (100%), 68 of 68 limbs after 6 months (100%), and 46 of 46 limbs after 1 year (100%). No limbs showed recanalization during the follow-up period of 12 months. Table 2 summarizes the closure rates. Fig. 1 shows the Kaplan-Meier survival curve analysis of the occlusion rates of incompetent GSV after RFA and CAC.
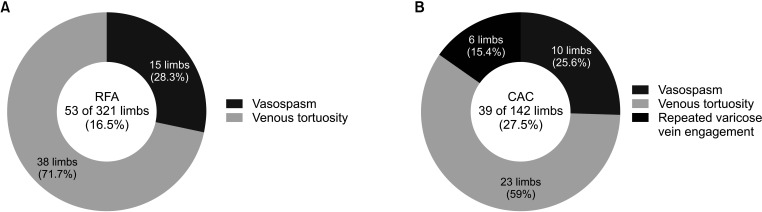
For RFA, a guidewire was used in 16.5% of cases (53 of 321 limbs). Reasons for hydrophilic guidewire usage were tortuosity (71.7%, 38 of 53 limbs) and vasospasm (28.3%, 15 of 53 limbs) (Fig. 2A). Catheter advancement was successfully achieved with a 0.025-inch guidewire in 79.2% of cases (42 of 53 limbs) and with a 0.018-inch guidewire in 20.8% of cases (11 of 53 limbs). RFA was successfully performed with a 0.025-inch guidewire in all 25 limbs that required the hydrophilic guidewire when treating a unilateral GSV lesion or the first of the bilateral GSV lesions (initial limb). However, the 0.025-inch guidewire was sufficient for only 17 of 28 limbs that required the hydrophilic guidewire when treating the second of the bilateral GSV lesions (subsequent limb). We had no choice but to use a 0.018-inch guidewire for the remaining 11 of 28 limbs. There was a statistically significant difference between the initial limb and the subsequent limb treatment in terms of the guidewire diameter (P < 0.001).
For CAC, a 0.035-inch hydrophilic guidewire was used in 27.5% of cases (39 of 142 limbs) where the passage of the J-wire guidewire was not achieved. Reasons for hydrophilic guidewire usage were tortuosity (59.0%, 23 of 39 limbs), vasospasm (25.6%, 10 of 39 limbs), and repeated engagement of a J-tip guidewire into the varicose tributaries (15.4%, 6 of 39 limbs) (Fig. 2B). In CAC, the frequency of hydrophilic guidewire usage was higher than that in RFA (P = 0.006).
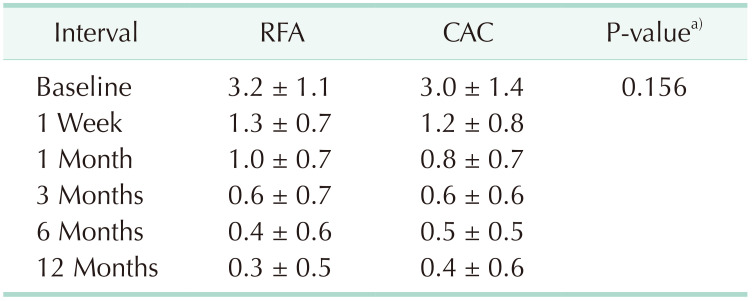
All 463 varicose vein treatment sessions were technically successful. VCSS scores at one week after the procedure and thereafter were significantly (P < 0.001) lower than baseline scores in both RFA and CAC groups. Moreover, there was no significant difference in VCSS between the 2 groups at all follow-up visits (Table 3).
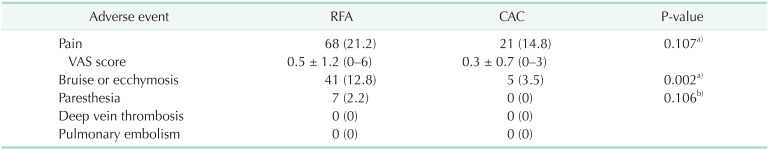
The common adverse events were pain over the affected treatment site, noted in 89 of 463 limbs (19.2%), and bruise (9.9%; 46 of 463 limbs). The bruise was less frequent after CAC compared with RFA (P = 0.002). Among the 464 limbs, paresthesia was noted in only 7 limbs that underwent RFA. There were no serious complications, such as deep vein thrombosis or pulmonary embolism (Table 4).
Go to : 
Technical success rates of endovenous treatment of the incompetent GSV were 95%–100% in several large-scale studies [151617]. However, several factors lower the technical success rate. These factors include difficulty in cannulation of the target vein [1518], usually because of a small diameter or vasospasm of the target vein. This problem can be overcome by use of a tourniquet, a more proximal puncture, and open access. Another factor is difficulty in visualization of the tip of the catheter on the US scan. If the tip is not visualized on longitudinal imaging, the transducer should be rotated to a cross-sectional view. However, even with this method, it is occasionally difficult to accurately locate the tip with US [19]. In these cases, the solution can be to use a venogram under fluoroscopy guidance [15]. The most challenging factor is difficulty in catheter advancement up to the SFJ. Catheter advancement can be difficult for several reasons. In this study, such reasons were tortuosity or vasospasm of the vein being treated and repeated engagement of the J-tip guidewire into the varicose tributaries.
To overcome the difficulty in catheter advancement up to the SFJ, some previous studies have suggested several good strategies. US-guided compression to negotiate catheter passage may be an option [18], but it may require a skillful operator who can manipulate a US probe with ease. Otherwise, US-guided compression can be time-consuming and yield a poor outcome. Additionally, several studies reported conversion to other methods, such as making additional venous access sites after failure with the first venous access [20].
Hydrophilic guidewire passage under fluoroscopic guidance provides a great level of comfort on the operator’s part, particularly if the operator is experienced with catheter and hydrophilic guidewire manipulation under fluoroscopic guidance. Perosi et al. [20] reported that successful fluoroscopy-guided retrograde access to the target vein can be achieved when antegrade access is impossible. However, these strategies expose both the patient and operator to unnecessary ionizing radiation. Furthermore, since the endovenous procedure for chronic venous insufficiency can be achieved only with the aid of ultrasonography from beginning to end, most of the clinics specializing in the treatment of varicose veins are not equipped with a fluoroscopy machine. A study has already been reported that the treatment of venous insufficiency of small saphenous veins using only ultrasonography after retrograde access is comparable to antegrade access in terms of safety and effectiveness [21]. If possible, it is desirable to conduct the procedure without the aid of fluoroscopy during endovenous treatment.
Making additional venous access is another option, if the target vein cannot be approached through the first venous access. Perosi et al. [20] reported a retrograde puncture of the GSV near the SFJ, while Hao et al. [22] reported an antegrade puncture of GSV just above the level of GSV tortuosity. Both methods proved to be effective, with a technical success rate of 100%. In cases of GSV obstruction or severe tortuosity where hydrophilic guidewire passage cannot be achieved, an additional venous access point may be a good solution. However, additional venous access results in a prolonged procedure time and additional patient discomfort. We believe that this double-puncture technique should be reserved for cases where hydrophilic guidewire passage cannot be achieved. In the present study, a total of 463 GSVs were treated with RF ablation or CAC. Ninety-two limbs were treated with the assistance of hydrophilic guidewire only, while we did not use other approaches, such as US-guided compression, fluoroscopy guidance, or additional venous accesses.
In the present study, the frequency of using a hydrophilic guidewire was higher in the CAC procedure when catheter advancement was not achieved. However, it is presumed that this is not due to the procedural characteristics of RFA and CAC, but a result according to the location of the puncture. In RFA, the puncture was performed at the knee joint level, whereas in CAC, the puncture was performed at the calf level. Therefore, the probability of encountering the tortuous segment of the GSV is high, and the probability that the J-wire guidewire would be headed toward the varicose tributaries is also high. In addition, since GSV at the calf level naturally has a smaller diameter compared to the GSV at the knee joint level, the difficulty of access is higher. As the difficulty of the puncture increases, the likelihood of venous spasm increases.
For RFA using the ClosureFast device in the present study, of the 53 cases that required the assistance of a hydrophilic guidewire, 11 cases required a 0.018-inch guidewire. Notably, in all 11 cases, the subsequent limbs and not the initial limbs were treated, and the reason for 0.018-inch guidewire usage was difficulty in 0.025-inch guidewire passage through the RF catheter. We suspect that this result suggests a potential obstacle such as carbonized blood product which impedes 0.025-inch guidewire passage after the initial limb treatment. However, this study does not provide solid evidence as to what causes the 0.025-inch guidewire passage difficulty. Considering these results, if both GSVs are treated with hydrophilic guidewires, it would be better to use a 0.018-inch guidewire first in the subsequent limb.
There are several limitations to this study. First, this was a retrospective study conducted at a single institute. Second, this study did not compare the usage of a hydrophilic guidewire with the other methods mentioned above. The lack of comparison with other methods at our institute is due to the fact that catheter advancement to the SFJ could be achieved with hydrophilic guidewire usage in the treatment of all troublesome 92 limbs from 463 limbs. The primary strategy at our institute is hydrophilic guidewire usage under US guidance because other options may be time-consuming or expose patients to unnecessary pain or ionizing radiation.
In conclusion, the usage of a hydrophilic guidewire could facilitate better catheter advancement when it is hindered because of vasospasm, tortuosity of the GSV, or repeated engagement of a J-tip guidewire into the varicose tributaries. As for RFA, the usage of a 0.018-inch guidewire could provide better catheter advancement when a 0.025-inch guidewire through RF catheter passage is troublesome.
Go to : 
Notes
Fund/Grant Support: The study was supported by a grant from the Terumo Research Fund (2017) through the Korean Society of Interventional Radiology.
Conflicts of Interest: Although a grant was from Terumo Research Fund through the Korean Society of Interventional Radiology, the funder has no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No potential conflict of interest relevant to this article was reported.
Go to : 
References
1. Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003; 37:1047–1053. PMID: 12756353.

2. Schwarz T, von Hodenberg E, Furtwängler C, Rastan A, Zeller T, Neumann FJ. Endovenous laser ablation of varicose veins with the 1470-nm diode laser. J Vasc Surg. 2010; 51:1474–1478. PMID: 20347542.

3. Park I. Successful use of VenaSeal system for the treatment of large great saphenous vein of 2.84-cm diameter. Ann Surg Treat Res. 2018; 94:219–221. PMID: 29629358.

4. Singhal S, Uthappa MC. Endovascular management of varicose veins: a review of literature. J Clin Interv Radiol. 2019; 3:98–104.

5. Shah A, Lau C, Stavropoulos SW, Nemeth A, Soulen MC, Solomon JA, et al. Comparison of physician-rated performance characteristics of hydrophilic-coated guide wires. J Vasc Interv Radiol. 2008; 19:400–405. PMID: 18295700.

6. Kähler J, Köster R, Brockhoff C, Reimers J, Baldus S, Terres W, et al. Initial experience with a hydrophilic-coated guidewire for recanalization of chronic coronary occlusions. Catheter Cardiovasc Interv. 2000; 49:45–50. PMID: 10627365.

7. Poncyliusz W, Falkowski A, Walecka A. Does use of hydrophilic guidewires significantly improve technical success rates of peripheral PTA? Med Sci Monit. 2004; 10 Suppl 3:55–57. PMID: 16538201.
8. Hartnell GG, Jones AM, Murphy P. Do hydrophilic guidewires affect the technical success rates of percutaneous angioplasty? Angiology. 1995; 46:229–234. PMID: 7879963.

9. Koizumi J, Mouri M, Watanabe M, Hiramatsu K. Transbrachial selective pulmonary angiography using a new 4 Fr curved pigtail catheter and hydrophiliccoated guidewire. Cardiovasc Intervent Radiol. 1998; 21:347–349. PMID: 9688808.

10. McCarthy JH, Miller GL, Laurence BH. Cannulation of the biliary tree, cystic duct and gallbladder using a hydrophilic polymer-coated steerable guide wire. Gastrointest Endosc. 1990; 36:386–389. PMID: 2210282.

11. Serrano A, Bravo-Balado A, Díaz AM, Barco-Castillo C, Trujillo CG. How can I get a renal access if I do not have an ultrasound and cannot opacify the collecting system? Another use of the hydrophilic guide wire. Ther Adv Urol. 2019; 11:1756287219868603. PMID: 31452687.

12. Oyama N, Urasawa K, Sakai H, Kitabatake A. Side branch protection with hydrophilic polymer coated guide wire during cutting balloon angioplasty of a bifurcated lesion. Jpn Heart J. 2003; 44:565–573. PMID: 12906038.

13. Joseph R, Huber M, Leeson B, Leeson K. Ultrasound-guided placement of a foley catheter using a hydrophilic guide wire. Clin Pract Cases Emerg Med. 2018; 2:143–146. PMID: 29849285.

14. Blitz BF. A simple method using hydrophilic guide wires for the difficult urethral catheterization. Urology. 1995; 46:99–100. PMID: 7604486.

15. Park SW, Yun IJ, Hwang JJ, Lee SA, Kim JS, Chang SH, et al. Fluoroscopy-guided endovenous foam sclerotherapy using a microcatheter in varicose tributaries followed by endovenous laser treatment of incompetent saphenous veins: technical feasibility and early results. Dermatol Surg. 2009; 35:804–812. PMID: 19389098.

16. Lurie F, Creton D, Eklof B, Kabnick LS, Kistner RL, Pichot O, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003; 38:207–214. PMID: 12891099.

17. Badri H, Bhattacharya V. A review of current treatment strategies for varicose veins. Recent Pat Cardiovasc Drug Discov. 2008; 3:126–136. PMID: 18537763.

18. Joh JH, Kim WS, Jung IM, Park KH, Lee T, Kang JM, et al. Consensus for the treatment of varicose vein with radiofrequency ablation. Vasc Specialist Int. 2014; 30:105–112. PMID: 26217628.

19. Disselhoff BC, der Kinderen DJ, Moll FL. Is there recanalization of the great saphenous vein 2 years after endovenous laser treatment? J Endovasc Ther. 2005; 12:731–738. PMID: 16363903.

20. Perosi NA, Johnson MG, Berkmen T. Fluoroscopic-guided approaches to radiofrequency vein ablation. J Vasc Interv RaKyosoo diol. 2013; 24:43–46.

21. Kim JS, Park SW, Yun IJ, Hwang JJ, Lee SA, Chee HK, et al. Retrograde endovenous laser ablation through saphenopopliteal junctional area for incompetent small saphenous vein: comparison with antegrade approach. Korean J Radiol. 2016; 17:364–369. PMID: 27134525.

22. Hao S, Cox S, Monahan TS, Sarkar R. Double prepuncture as a valuable adjunctive technique for complex endovenous ablation. J Vasc Surg Venous Lymphat Disord. 2017; 5:507–513. PMID: 28623986.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download