Abstract
Poor graft function (PGF) is a serious, potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation. Eltrombopag has shown multilineage responses in patients with refractory severe aplastic anemia, supporting the idea that it may improve cytopenia in patients with PGF. This retrospective, single center analysis included 8 Korean patients receiving eltrombopag for PGF. Median interval between transplant and eltrombopag treatment was 73 days, and the median duration treatment was 3.5 weeks. With median maximum daily dose of 50 mg, the time to best response was 93 days. Median hemoglobin increased from 8.2 g/dL to 10.9 g/dL, platelet from 18.5 × 109/L to 54 × 109/L, and absolute neutrophil count from 1.25 × 109/L to 3.32 × 109/L. In conclusion, eltrombopag is a good option for PGF in Korean patients, even at a lower dose compared to western patients.
Graphical Abstract
Poor graft function (PGF) is a serious, potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation (alloHSCT). After high-dose chemo- or radiotherapy based conditioning regimen, engraftment of hematopoietic stem cell and normalization of blood counts within 3-4 weeks from alloHSCT is expected.1 PGF is defined as cytopenia in at least 2 lineages (platelet < 20 × 109/L, absolute neutrophil count < 0.5 × 109/L, hemoglobin < 7.0 g/dL), and/or with transfusion requirement beyond day +28 post-alloHSCT, with full donor chimerism and without relapse or severe graft-versus-host disease (GVHD).2 Primary PGF refers to incomplete engraftment, while secondary PGF is defined as a loss of initial engraftment. PGF is reported in 5–25% of alloHSCT recipients and is associated with increased mortality and morbidity.3 Therapies for PGF include transfusion, intravenous immunoglobulin, granulocyte colony stimulating factor, stem cell boost, and second transplantation. However, these therapies are either only partially effective or not readily available and conveys significant adverse events (AEs).45 Eltrombopag is an oral, non-peptide, small-molecule, thrombopoietin receptor agonist that is widely used for treatment of immune thrombocytopenia (ITP) and severe aplastic anemia.6 Recently, eltrombopag has shown trilineage responses in some patients with refractory severe aplastic anemia,7 supporting the idea that it may improve cytopenia in patients with PGF. Subsequently, a handful of studies have successfully evaluated the role of eltrombopag to treat PGF primarily in western patients.1891011 On the other hand, it is widely accepted that East Asian patients require less eltrombopag dose.12 As such, we attempted to explore the efficacy of eltrombopag in PGF treatment in Korean patients.
This was a retrospective, single center study carried out at Seoul National University Hospital. Eight patients receiving eltrombopag for PGF after alloHSCT between July 2018 to April 2021 were identified and their medical records were reviewed and analyzed for demographics, disease characteristics, treatment, and clinical course. The AEs were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
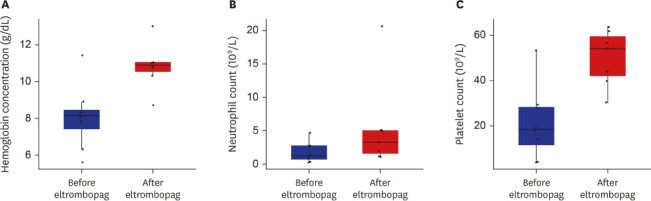
The baseline clinical characteristics including donor type, conditioning regimen/intensity, the amount of CD34 cells infused are summarized in Table 1. Two patients received upfront transplantation without prior induction chemotherapy, 3 patients received transplantation after prior chemotherapy and during remission, and the other 3 received transplantation as a salvage therapy due to graft failure or disease progression. Median age at transplant was 46 years (range, 18–73 years). Median CD34 infusion dose was 4.5 × 106 /kg (range, 0.31–5.9 × 106/kg). Median interval between transplant and start of eltrombopag treatment was 73 days (range, 23–477 days). Median duration of eltrombopag treatment was 3.5 weeks (range, 1–27 weeks) and the median maximum daily dose was 50 mg (range, 25–75 mg). Four of the recipients started at a dose of 25 mg, and the other four started at a dose of 50 mg. The maximal administration dose was 25 mg for two patients, 50 mg for five patients, and 75 mg for one patient. All but one patient were transfusion-dependent at the time of eltrombopag prescription. One patient died due to pneumonia during cytopenic period, thus was excluded from efficacy analyses. Median hemoglobin concentration at the start of eltrombopag administration was 8.2 g/dL (range, 5.6–11.4 g/dL), median neutrophils 1.26 × 109/L (range, 0.28–4.7 × 109/L), and platelets 18.5 × 109/L (range, 4–53 × 109/L). Responsiveness was defined as platelet recovery to ≥ 50 × 109/L without transfusion for > 7 consecutive days.9 The median time to best response from the first day of eltrombopag administration was 93 days (range, 28–105 days). Median hemoglobin concentration at best response was 10.9 g/dL (range, 8.7–13.0 g/dL), median neutrophils 3.32 × 109 /L (range, 1.2–20.6 × 109/L), and median platelets 54 × 109/L (range, 30–64 × 109/L). Fig. 1 graphically illustrates the improvement in hemogram after the use of eltrombopag. Unplanned eltrombopag discontinuation was noted in 1 patient due to concerns regarding GVHD. There were no eltrombopag-related deaths or grade 3/4 toxicities nor reports of cataract, thrombosis, or bone marrow fibrosis. There were 3 events of grade 2 liver enzyme elevations, which resolved with conservative management.
Eltrombopag induces differentiation of CD34+ hematopoietic precursor cells into committed CD41+ megakaryocyte progenitor cells and stimulates the proliferation of megakaryocyte progenitor cells.13 Furthermore, eltrombopag stimulates c-MPL receptors and improves hematopoiesis at stem cell level. Aled et al.’s experimental investigation using thrombopoietin provides evidence to this drug mechanism. Through in vitro and in vivo data using mice, it was proved that MPL expression in hematopoietic stem cell correlates with megakaryocytic differentiation potential, indicating that thrombopoietin in the bone marrow is associated with maintaining megakaryocytic differentiation in hematopoietic stem and progenitor cells. Also, the data showed that thrombopoietin is required for the proliferation of hematopoietic stem and progenitor cells with megakaryopoietic potential.14 Based on this mechanism, eltrombopag, which is a thrombopoietin receptor agonist, is expected to be able to control PGF. Our report is important in that 1) to the best of our knowledge, this is the first report of eltrombopag treatment for PGF in Korea; and 2) we provide further understanding for physicians to infer decision-making nuances regarding appropriate and realistic eltrombopag use. Specifically, it has been well-delineated that there is inter-ethnic differences in pharmacokinetics of eltrombopag metabolism.15 Due to the increased plasma exposure to eltrombopag in East Asian patients compared to non-East Asians, eltrombopag is recommended at a lower dose in East Asian ITP patients.1216 Current recommended starting eltrombopag dose is 25 mg/day for East Asian ITP patients versus 50 mg/day for non-East Asian ITP patients. In the case of PGF patients, although the standardized starting dose has not been established, previous studies have shown that East Asian patients can attain similar effects at a lower dose eltrombopag compared to Caucasians. In a case series conducted by Tang et al.10 in China which enrolled 12 patients, the maximum daily dose of eltrombopag for PGF patients was 25–75 mg, and in a case series conducted by Tanaka et al.17 in Japan, which also enrolled 12 patients the maximum daily dose was 12.5–50 mg. On the other hand, in a Spanish case series conducted by Rivera et al.18 which included 14 patients, the maximum daily dose was 50–150 mg, and in an Italian case series conducted by Marotta et al.1 which included 12 patients, the maximum dose was also 50–150 mg. Although these studies were retrospective case series, it raises high possibility that East Asians could respond to lower dose eltrombopag compared to Caucasians for PGF, as is consistent with ITP. In our study including 8 Korean patients, the maximum daily dose was 25–75 mg which was lower than the aforementioned western case series, and at which we proved the efficacy and safety of eltrombopag.
In conclusion, we showed that eltrombopag induces sustained response in Korean patients with both primary and delayed PGF after alloHSCT and that required dose of eltrombopag is lower in East Asians.
ACKNOWLEDGMENTS
The results of this study have been submitted to 72nd Annual Meeting of the Korean Association of Internal Medicine, 2021, Seoul, Republic of Korea.
Notes
Author Contributions:
Conceptualization: Byun JM.
Data curation: Ahn HJ, Byun JM, Kim I, Youk J, Koh Y, Shin DY, Hong J, Yoon SS.
Formal analysis: Ahn HJ, Byun JM.
Investigation: Ahn HJ.
Methodology: Byun JM.
Supervision: Byun JM.
Writing - original draft: Ahn HJ, Byun JM.
Writing - review & editing: Byun JM, Kim I, Youk J, Koh Y, Shin DY, Hong J, Yoon SS.
References
1. Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54(8):1346–1353. PMID: 30679824.
2. Kong Y. Poor graft function after allogeneic hematopoietic stem cell transplantation-an old complication with new insights. Semin Hematol. 2019; 56(3):215–220. PMID: 31202433.
3. Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation - frequency and outcomes. Bone Marrow Transplant. 2004; 33(7):729–734. PMID: 14755315.
4. Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9):1440–1443. PMID: 24862637.
5. Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014; 23(9):1087–1098. PMID: 23294601.
6. Wong RS, Saleh MN, Khelif A, Salama A, Portella MS, Burgess P, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017; 130(23):2527–2536. PMID: 29042367.
7. Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012; 367(1):11–19. PMID: 22762314.
8. Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020; 26(3):e65–e73. PMID: 31830528.
9. Bento L, Bastida JM, García-Cadenas I, García-Torres E, Rivera D, Bosch-Vilaseca A, et al. Thrombopoietin receptor agonists for severe thrombocytopenia after allogeneic stem cell transplantation: experience of the Spanish Group of Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019; 25(9):1825–1831. PMID: 31152794.
10. Tang C, Chen F, Kong D, Ma Q, Dai H, Yin J, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J Hematol Oncol. 2018; 11(1):103. PMID: 30115080.
11. Halahleh K, Gale RP, Da’na W, Ma’koseh M, Saadeh S, Alan W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant. 2021; 56(1):4–6. PMID: 32572137.
12. Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ, et al. Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia. Blood Res. 2015; 50(1):19–25. PMID: 25830126.
13. Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009; 27(2):424–430. PMID: 19038790.
14. O’Neill A, Chin D, Tan D, Abdul Majeed AB, Nakamura-Ishizu A, Suda T. Thrombopoietin maintains cell numbers of hematopoietic stem and progenitor cells with megakaryopoietic potential. Haematologica. 2021; 106(7):1883–1891. PMID: 32527954.
15. Shida Y, Takahashi N, Nohda S, Hirama T. Pharmacokinetics and pharmacodynamics of eltrombopag in healthy Japanese males. Jpn J Clin Pharmacol Ther. 2011; 42(1):11–20.
16. Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011; 51(6):842–856. PMID: 20663993.
17. Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016; 22(5):919–924. PMID: 26785333.
18. Rivera D, Bastida JM, Lopez-Corral L, Sanchez-Guijo F, Cabrero M, Martin A, et al. Usefulness of eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2019; 54(5):757–761. PMID: 30356164.
Fig. 1
Complete blood cell count before and after eltrombopag treatment. (A) Median hemoglobin concentration increased from 8.2 g/dL to 10.9 g/dL. (B) Median absolute neutrophil count from 1.25 × 109/L to 3.32 × 109/L. (C) Median platelet increased from 18.5 × 109/L to 54 × 109/L.
Table 1
Clinical characteristics
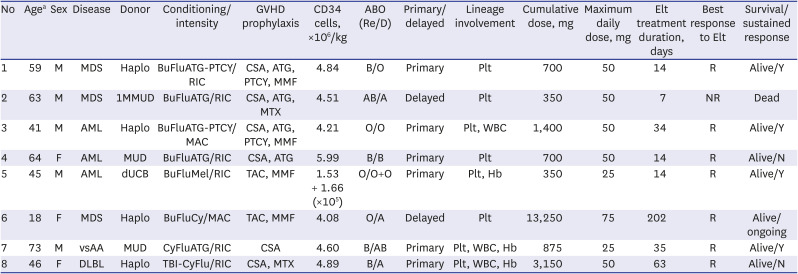
GVHD = graft-versus-host disease, Re = recipient, R = responsive, D = donor, Elt = eltrombopag, M = male, MDS = myelodysplastic syndrome, haplo = haplo-identical, PTCY = post-cyclophosphadmide, RIC = reduced intensity conditioning, CSA = cyclosporin, ATG = antithymocyte globulin, MMF = mycophenolate mofetil, Plt = platelet, 1MMUD = 1 mismtached unrelated donor, MTX = methotrexate, NR = no response, AML = acute myeloid leukemia, MAC = myeloablative conditioning, WBC = white blood cell, F = female, MUD = matched unrelated donor, dUCB = double umbilical cord blood, TAC = tacrolimus, Hb = hemoglobin, vsAA = very severe aplastic anemia, DLBL = diffuse large B-cell lymphoma.
aAge at allogeneic hematopoietic stem cell transplantation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download