INTRODUCTION
The fact that there are many types of diagnostic assays for
Clostridioides difficile infection (CDI) suggests that CDI diagnosis is difficult. Moreover, distinguishing the patients from asymptomatic carriers is challenging. Asymptomatic carriers may be a source of infection; therefore, establishing an appropriate CDI diagnosis system is essential to improve the quality management of CDI, thereby rendering treatment, prevention, and infection control appropriate.
12 Additionally, it can provide meaningful data that can be compared and evaluated based on the homogeneity of CDI diagnostic criteria for epidemiologic studies.
There are various diagnostic assays for CDI including
C. difficile culture, detection of toxin genes for toxin A, toxin B, and toxin A, B (
tcdA,
tcdB), and glutamate dehydrogenase (GDH) secreted by
C. difficile strains; detection techniques include cell culture, latex agglutination, immunochromatography (IC), enzyme immunoassay (EIA), nucleic acid amplification test (NAAT), cell cytotoxicity neutralization assay (CCNA), and toxigenic culture. The biggest change in diagnostic techniques since 2010 is that various NAATs, such as polymerase chain reaction, have been developed to detect genes of toxin A, toxin B, or binary toxins, thereby improving the sensitivity of CDI tests through novel molecular diagnostic techniques.
1234
Guidelines for CDI diagnosis have been made by European Society of Clinical Microbiology and Infectious Disease (ESCMID) group,
5 Infectious Diseases Society of America (IDSA)/Society for Healthcare Epidemiology of America (SHEA),
6 American College of Gastroenterology (ACG)
7 and American Academy of Pediatrics (APP).
8 Some recommendations add endoscopy findings to the CDI diagnostic criteria,
267 In Korea, clear CDI diagnostic standards are not established yet, so each institution prepares and uses their own diagnostic standards.
The authors first conducted a national survey on CDI diagnosis in 2015.
9 Since then, the interest in CDI has increased, and the choice of assays became wider with several commercialized GDHs introduced in Korea. Therefore, this study conducted a survey of the domestic CDI assays with more varied questions than the 2015 study to understand the current situation in Korea. By this, we aimed to provide necessary evidence in setting the criteria for CDI diagnosis and treatment, infection control, and epidemiologic studies.
Go to :

RESULTS
Current status of CDI assays
One-hundred and fifty institutions responded to the questionnaire, of which 90 (60.0%) usually performed CDI assays. When subdivided by institution types, all tertiary hospitals (n = 38) as well as 46.4% (51/110) of general hospitals performed CDI assays, suggesting that tertiary hospitals perform CDI assays at a statistically higher rate (P < 0.001). One of the institutions that responded positively to conducting CDI assays was excluded from further analysis as it was a commercial laboratory. The institutions that responded were distributed nationwide (Seoul 24, Incheon 8, Busan 15, Daegu 7, Gwangju 7, Daejeon 5, Ulsan 2, Gyeonggi 36, Chungbuk 7, Chungnam 6, Jeonbuk 8, Jeonnam 7, Gyeongbuk 3, Gyeongnam 8). By region, a significant number of institutions performed CDI assays in Seoul (87.5%), and the number of institutions was relatively lower in Gyeongnam (25.0%). The biggest reasons for not performing CDI assays were that there were few requests for testing, thus tests were performed by the delegated institutions (70.0%) and there was no request for testing (18.3%). Other reasons included lack of manpower and equipment for the assay.
Analyzing the characteristics of the patients referred for CDI assays showed that 52.4% (n = 44) of the institutions performed CDI assays on the patients with acute or chronic diarrhea and a history of antibiotic use, which was significantly higher than the institutions that performed CDI assays on the patients with diarrhea alone (38.1%). In terms of departments, the assays were requested from the department of gastroenterology alone or departments of internal medicine and surgery including gastroenterology in 57 institutions, from departments of internal medicine excluding gastroenterology and infectious diseases in 63 institutions, from the department of infectious diseases alone or internal medicine and surgery including infectious diseases in 26 institutions, and from departments of surgery in 32 institutions. This suggests that a significantly higher number of assays were requested by departments of gastroenterology and internal medicine. Similar trends were observed in terms of departments in which patients were diagnosed with CDI. Referral analysis of CDI assays in pediatric patients showed that 62.8% (n = 54) practitioners did not perform CDI assays (including for those under 2 years of age).
A survey on a sample rejection criterion based on the Bristol stool chart showed that 27 (30.3%) institutions actually referred to the Bristol stool chart. However, most of these institutions only referred to the chart during the assay, and only 9 (10.2%) said they rejected diarrheal stool. This suggests that since there are difficulties in rejecting inappropriate samples, from failure to establish sample rejection criteria to challenges rejecting samples for tests prescribed by a referring clinician, when receiving a sample, all tests were conducted when requested.
CDI diagnostic test methods
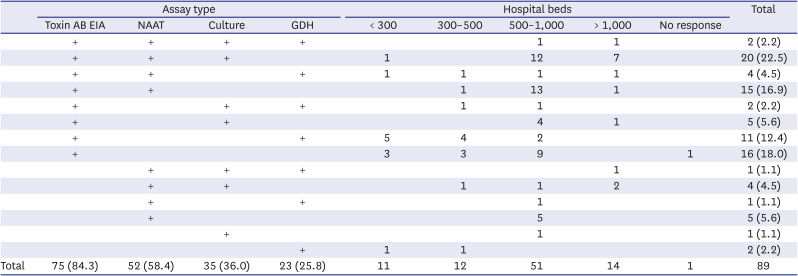
Table 2 summarizes the survey results on whether toxin AB EIA, NAAT,
C. difficile culture, GDH, and CCNA were conducted as CDI diagnostic tests according to hospital size. No institutions in Korea responded that CCNA was performed. For those who conducted two or more diagnostic tests among the 69 institutions that responded, 30 performed the tests simultaneously, and 39 conducted them sequentially, though the difference was not significant, and it was difficult to analyze certain rules or patterns of sequential tests.
Table 3 summarizes the details of each CDI assay. The number of specimens examined for CDI diagnosis according to assay types and hospital size is shown in
Table 4, and turnaround time (TAT) of each CDI diagnostic method is summarized in
Table 5.
Table 2
Combinations of assay types for diagnosis of Clostridioides difficile infection according to hospital size
|
Assay type |
Hospital beds |
Total |
|
Toxin AB EIA |
NAAT |
Culture |
GDH |
< 300 |
300–500 |
500–1,000 |
> 1,000 |
No response |
|
+ |
+ |
+ |
+ |
|
|
1 |
1 |
|
2 (2.2) |
|
+ |
+ |
+ |
|
1 |
|
12 |
7 |
|
20 (22.5) |
|
+ |
+ |
|
+ |
1 |
1 |
1 |
1 |
|
4 (4.5) |
|
+ |
+ |
|
|
|
1 |
13 |
1 |
|
15 (16.9) |
|
+ |
|
+ |
+ |
|
1 |
1 |
|
|
2 (2.2) |
|
+ |
|
+ |
|
|
|
4 |
1 |
|
5 (5.6) |
|
+ |
|
|
+ |
5 |
4 |
2 |
|
|
11 (12.4) |
|
+ |
|
|
|
3 |
3 |
9 |
|
1 |
16 (18.0) |
|
|
+ |
+ |
+ |
|
|
|
1 |
|
1 (1.1) |
|
|
+ |
+ |
|
|
1 |
1 |
2 |
|
4 (4.5) |
|
|
+ |
|
+ |
|
|
1 |
|
|
1 (1.1) |
|
|
+ |
|
|
|
|
5 |
|
|
5 (5.6) |
|
|
|
+ |
|
|
|
1 |
|
|
1 (1.1) |
|
|
|
|
+ |
1 |
1 |
|
|
|
2 (2.2) |
|
Total |
75 (84.3) |
52 (58.4) |
35 (36.0) |
23 (25.8) |
11 |
12 |
51 |
14 |
1 |
89 |

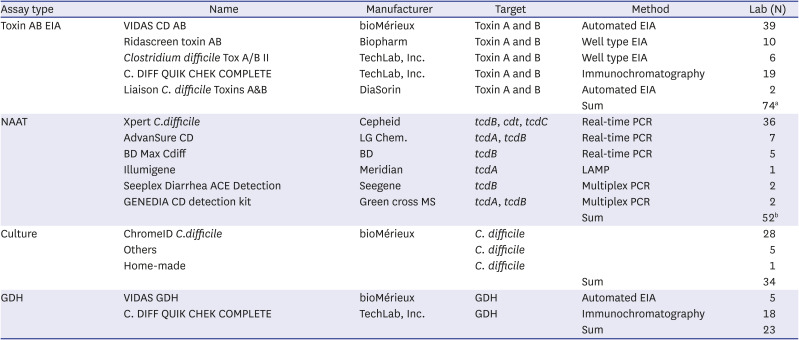
Table 3
Clostridioides difficile assay methods and the numbers of laboratories that participated in the survey
|
Assay type |
Name |
Manufacturer |
Target |
Method |
Lab (N) |
|
Toxin AB EIA |
VIDAS CD AB |
bioMérieux |
Toxin A and B |
Automated EIA |
39 |
|
Ridascreen toxin AB |
Biopharm |
Toxin A and B |
Well type EIA |
10 |
|
Clostridium difficile Tox A/B II |
TechLab, Inc. |
Toxin A and B |
Well type EIA |
6 |
|
C. DIFF QUIK CHEK COMPLETE |
TechLab, Inc. |
Toxin A and B |
Immunochromatography |
19 |
|
Liaison C. difficile Toxins A&B |
DiaSorin |
Toxin A and B |
Automated EIA |
2 |
|
|
|
Sum |
74a
|
|
NAAT |
Xpert C.difficile
|
Cepheid |
tcdB, cdt, tcdC
|
Real-time PCR |
36 |
|
AdvanSure CD |
LG Chem. |
tcdA, tcdB
|
Real-time PCR |
7 |
|
BD Max Cdiff |
BD |
tcdB
|
Real-time PCR |
5 |
|
Illumigene |
Meridian |
tcdA
|
LAMP |
1 |
|
Seeplex Diarrhea ACE Detection |
Seegene |
tcdB
|
Multiplex PCR |
2 |
|
GENEDIA CD detection kit |
Green cross MS |
tcdA, tcdB
|
Multiplex PCR |
2 |
|
|
|
Sum |
52b
|
|
Culture |
ChromeID C.difficile
|
bioMérieux |
C. difficile
|
|
28 |
|
Others |
|
C. difficile
|
|
5 |
|
Home-made |
|
C. difficile
|
|
1 |
|
|
|
Sum |
34 |
|
GDH |
VIDAS GDH |
bioMérieux |
GDH |
Automated EIA |
5 |
|
C. DIFF QUIK CHEK COMPLETE |
TechLab, Inc. |
GDH |
Immunochromatography |
18 |
|
|
|
Sum |
23 |

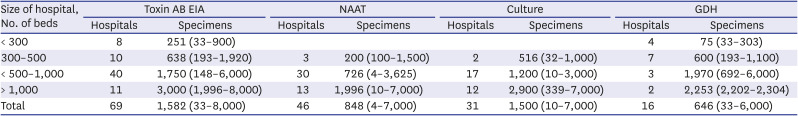
Table 4
Numbers of specimens examined for CDI diagnosis according to assay types and hospital size per month in 2018
|
Size of hospital, No. of beds |
Toxin AB EIA |
NAAT |
Culture |
GDH |
|
Hospitals |
Specimens |
Hospitals |
Specimens |
Hospitals |
Specimens |
Hospitals |
Specimens |
|
< 300 |
8 |
251 (33–900) |
|
|
|
|
4 |
75 (33–303) |
|
300–500 |
10 |
638 (193–1,920) |
3 |
200 (100–1,500) |
2 |
516 (32–1,000) |
7 |
600 (193–1,100) |
|
< 500–1,000 |
40 |
1,750 (148–6,000) |
30 |
726 (4–3,625) |
17 |
1,200 (10–3,000) |
3 |
1,970 (692–6,000) |
|
> 1,000 |
11 |
3,000 (1,996–8,000) |
13 |
1,996 (10–7,000) |
12 |
2,900 (339–7,000) |
2 |
2,253 (2,202–2,304) |
|
Total |
69 |
1,582 (33–8,000) |
46 |
848 (4–7,000) |
31 |
1,500 (10–7,000) |
16 |
646 (33–6,000) |

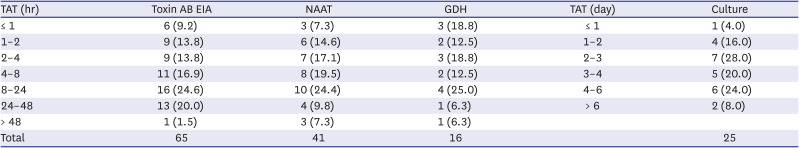
Table 5
Turnaround time for Clostridioides difficile assays of laboratories that participated in the survey
|
TAT (hr) |
Toxin AB EIA |
NAAT |
GDH |
TAT (day) |
Culture |
|
≤ 1 |
6 (9.2) |
3 (7.3) |
3 (18.8) |
≤ 1 |
1 (4.0) |
|
1–2 |
9 (13.8) |
6 (14.6) |
2 (12.5) |
1–2 |
4 (16.0) |
|
2–4 |
9 (13.8) |
7 (17.1) |
3 (18.8) |
2–3 |
7 (28.0) |
|
4–8 |
11 (16.9) |
8 (19.5) |
2 (12.5) |
3–4 |
5 (20.0) |
|
8–24 |
16 (24.6) |
10 (24.4) |
4 (25.0) |
4–6 |
6 (24.0) |
|
24–48 |
13 (20.0) |
4 (9.8) |
1 (6.3) |
> 6 |
2 (8.0) |
|
> 48 |
1 (1.5) |
3 (7.3) |
1 (6.3) |
|
|
|
Total |
65 |
41 |
16 |
|
25 |

The perception of the sensitivity and specificity of each CDI assay experienced by the laboratory medicine specialists is summarized in
Fig. 1. Assuming that the sensitivity of 80% reported positive perception and less than 60% reported negative perception, the sensitivity of toxin AB EIA was more negatively perceived, and that on specificity was more positively perceived. On NAAT, more than 90% and more than 80% showed positive perception on sensitivity and specificity, respectively. Positive responses accounted for more than 2/3 of the responses in regard to sensitivity and specificity of
C. difficile culture and GDH.
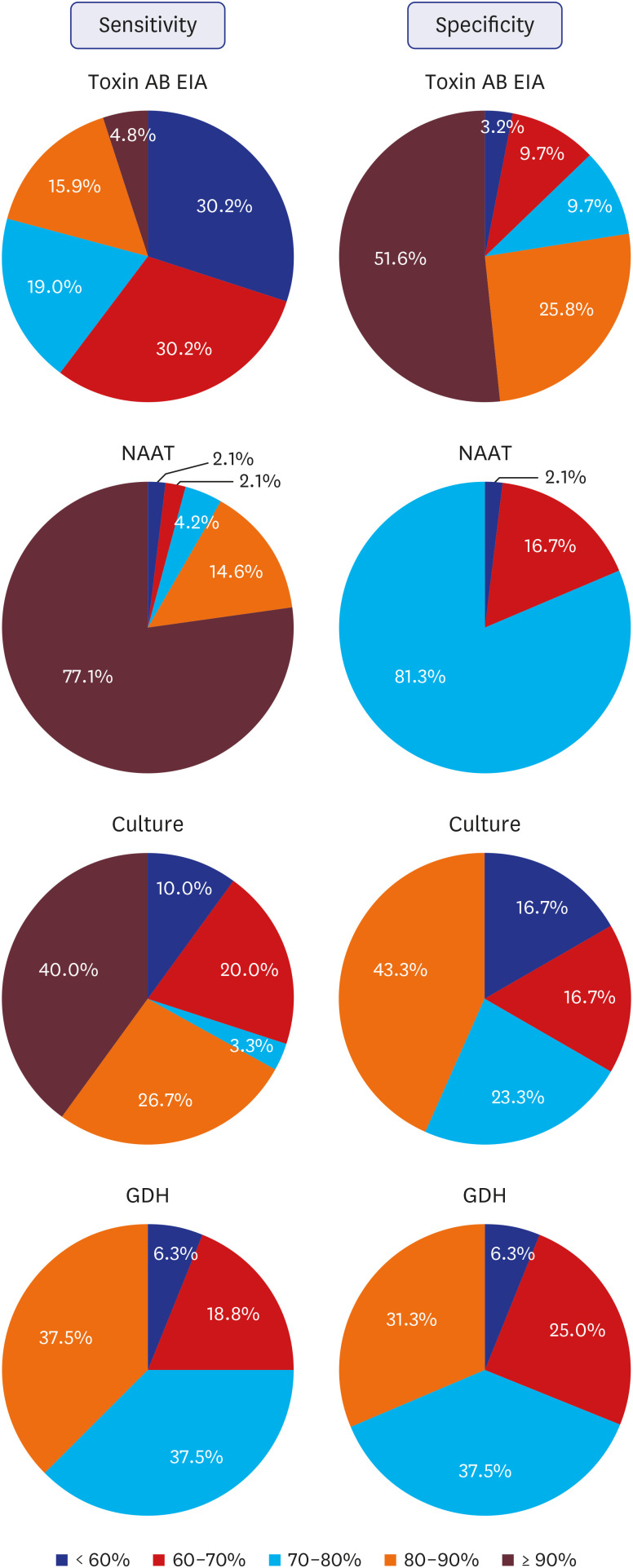 | Fig. 1
The perception of the sensitivity and specificity of each Clostridioides difficile assays experienced by the laboratory medicine specialists.
EIA = enzyme immunoassay, NAAT = nucleic acid amplification test, GDH = glutamate dehydrogenase.

|
Algorithm and stand-alone tests
Survey on the perception of algorithm tests showed that 22.1% (n = 19) responded algorithmic test was useful, 68.6% (n = 59) responded it was useful but in need of countermeasures, and 9.3% (n = 8) responded it was unnecessary. Statistically, those that said it was "useful" and "unnecessary" were significantly less than the other groups, and those that said it was useful but in need of countermeasures were significantly higher than the other groups (P < 0.001). If the algorithm test were conducted, 64.1% responded that GDH and toxin AB EIA would be tested first and if toxin comes out to be negative, NAAT would be conducted (GDH + toxin AB EIA → GDH(+)/toxin(−) → NAAT), and 29.5% responded that GDH and NAAT would be conducted first, and according to the results, toxin AB EIA would be conducted (NAAT or GDH → any(+) → Toxin AB EIA).
On conducting NAAT as a stand-alone assay, 8 institutions responded that it is the best stand-alone assay, and that additional test was unnecessary. Most of the other respondents said that, although NAAT is a highly sensitive and accurate test, due to insurance costs, economic feasibility, problems with manpower, and additional toxin AB EIA tests, algorithm test should be considered as a countermeasure rather than a stand-alone.
CDI surveillance for infection control
Sixty-three (73.3%) institutions responded that they performed surveillance on CDI. On the timing of quarantine, 62.3% (n = 38) responded that the criteria were based on the diarrhea symptoms and having one or more items of the CDI diagnostic test as positive, and 31.1% (n = 19) responded that only positive CDI test criteria were used, regardless of diarrhea symptoms. As for the timing to end quarantine, 75% (n = 39) responded it was based on the disappearance of diarrhea symptoms, and 25% (n = 13) responded to rely on the CDI test to end quarantine.
Go to :

DISCUSSION
The reason for difference in regional distribution of medical institutions that conduct CDI tests in Korea is that, in Seoul, 50% of such institutions were tertiary hospitals, suggesting that there were more institutions with facilities and infrastructure for CDI diagnostic testing and the interest in CDI was also high. In Gyeongnam, all 8 institutions that responded to the survey were general hospitals. The reasons for failure to perform the CDI assay were thought to be small size of the institution as well as lack of awareness regarding the necessity and magnitude of CDI assays in the clinic.
When the characteristics of the CDI assay-referred patients were analyzed, more than half of the respondents had diarrhea symptoms and a history of antibiotic use, suggesting that a significantly higher number of clinicians recognize the main cause of CDI as antibiotic-associated diarrhea. Furthermore, 38.1% of the patients were referred with diarrhea symptoms, and because it is difficult to distinguish C. difficile from other diarrhea-causative bacteria based on the symptoms alone, even if the history of antibiotic use is not clear, CDI assays were performed with just diarrhea symptoms. The department that mainly requested CDI assay was the department of gastroenterology, which is likely because the main symptom of CDI is diarrhea, which is of utmost interest to them. In addition, there were many referrals from departments of internal medicine excluding the gastroenterology and infectious diseases department, which is considered to be due to the fact that diarrhea is a common symptom in patients with various diseases. The rate of CDI testing in pediatric patients was higher than expected as 37.2% of the institutions conducted CDI tests in pediatric patients, suggesting that there is much interest in CDI in pediatric patients.
Diarrheal stool is recommended for CDI tests,
56 but the result of the survey was that many institutions do not reject non-diarrheal specimens in reality and perform the test on the non-diarrheal stools. This could be due to the facts that it is difficult to reject a referral from a clinician in regard to the clinical examination process in the hospitals, and it requires manpower and time. Thus, it is difficult to actually implement the specimen rejection criteria.
Survey on the CDI assay showed that 73.0% (65/89) institutions performed a combination of two or more assays, similar to the 2015 survey with 78.9% (45/57). Combining the use of toxin AB EIA, NAAT, and C. difficile culture was the most common practice (22.5%, n = 20), followed by using toxin AB EIA and NAAT together. The 2015 survey also showed that the most commonly used combination was the same (40.4%, n = 23), but the proportion became significantly lower in the 2018 survey (P = 0.036). In the 2018 survey, as the number of institutions that conducted CDI assays increased, and the number of institutions that combined the assays in different ways increased. Eleven (12.4%) institutions used the combination of toxin AB EIA and GDH, which was significantly increased from 1 (1.8%) institution in the 2015 survey. In particular, 9 of those institutions had less than 500 beds, suggesting that the combinatory use of toxin AB EIA and GDH increased mainly in small hospitals. Sixteen (18.0%) institutions used toxin AB EIA alone, the proportion was not significantly different from the 2015 survey (15.8%, n = 9).
In terms of the number of beds, the number of institutions that conducted CDI assay increased from the 2015 survey with 1 institution having 300–500 beds and 3 institutions having less than 300 beds. The institutions with fewer number of beds were more likely to use toxin AB EIA and GDH tests and less likely to use C. difficile culture and NAAT, which may be due to the arrangement of equipment and manpower.
Toxin AB EIA was the most used method for CDI assay (84.3%), and 89.5% of (51/57) institutions used this method in the 2015 survey, suggesting that it is still the most used method. Automated EIA equipment was most commonly used for toxin AB EIA, followed by a kit using the IC principle, suggesting that many medical institutions in Korea were capable of performing an automated EIA test, they wanted to use IC method to obtain the result relatively quickly, or they preferred to obtain the results from GDH/toxin AB simultaneously. NAAT was the next commonly used assay, and there was no significant difference in the usage proportion from the 2015 survey with 64.9% (37/57) of institutions.
About 40% of the institutions performed
C. difficile culture, which was significantly lower than the 64.9% of the institutions in the 2015 survey (
P = 0.004), and the absolute number of institutions conducting it decreased. The
C. difficile culture method, which simply checks the growth of
C. difficile strain but not the production of toxin, had limitations in diagnosing CDI because it could not identify toxigenic
C. difficile, and its TAT is longer than other CDI diagnostic tests. Similar to culture, GDH can detect the presence of
C. difficile regardless of toxin production, and it is thought to replace the
C. difficile culture, as it can detect
C. difficile with a sensitivity of 90% or more.
10 The most commonly used culture kit is ChromeID Agar (bioMérieux, Marcy-l'Étoile, France), which was the most commonly used in the 2015 survey as well, and it is preferred since it can screen the
C. difficile colony relatively well with color reaction.
The GDH was the least frequently used CDI assay with 23 (25.8%) institutions, but compared to the 2015 survey with only 1 institution, the number has significantly increased. The GDH was most recently introduced in Korea, so it is not used actively yet, but its use is gradually on the rise. C.DIFF QUICK CHECK COMPLETE kit (TechLab, Inc., Blacksburg, VA, USA) that uses IC principle was the most commonly used kit for GDH, which was thought to be due to its ability to test both GDH and toxin AB simultaneously.
Toxin AB EIA had the highest number of assays, but the number of assays conducted varied greatly depending on the size of the institution. NAAT was tested less frequently compared to toxin AB EIA, which may be linked to the size of each institution and the availability of equipment and trained personnel.
Results from toxin AB EIA and NAAT were relatively quick as reported by most institutions; about 80% of the institutions reported the test results on the same day, and those that did not report on the same day did the following day, suggesting that more than 90% of the institutions reported the results within 2 days. Recently in Korea, a new system was introduced that reports NAAT results within 6 hours upon referral with an added fee, which may have further shortened the TAT. This requires further intervention. In TAT of GDH, 90% of the institutions reported the results on the same day, leading to the highest rate of same-day reporting among CDI assays, and 80% of the institutions used the IC method, thereby rendering it easier.
In this study, we surveyed the perception of sensitivity and specificity of the CDI assays experienced by actual laboratory medicine specialists. Although data on this under well-planned research settings or those derived through meta-analysis are well-known, it is difficult to find a study on the sensitivity and specificity recognized by laboratory medicine specialists who perform and read these assays in actual clinical situations. Perceptions on sensitivity and specificity investigated in this study is said to be a good indicator of utility in situations where CDI assays are used.
The perception of sensitivity of toxin AB EIA was more negative than positive. The sensitivity of toxin AB EIA is low, such that the pooled sensitivity of toxin AB EIA was about 60% and may be less than that depending on the method.
510 The fact that perception by some specialists who actually perform the tests in Korea was positive could be due to the automated EIA method, which is the most commonly used method, and is more sensitive than other methods.
10 The perception of sensitivity and specificity of NAAT was mostly positive, suggesting that the specialists were quite satisfied, consistent with the fact that sensitivity and specificity of NAAT are known to be remarkable.
510 The relatively lower specificity of
C. difficile culture encountered by some could be due to the characteristics of the culture containing non-specific bacteria with a specificity of 60–80%. Perceptions on the sensitivity of GDH was relatively positive, which was lower than NAAT but higher than toxin AB EIA, and that on the specificity led to lower satisfaction compared to NAAT and toxin AB EIA. The lower specificity could be due to the fact that non-toxigenic
C. difficile may be detected via GDH in some cases.
Perception on the algorithm test projected it as useful but in need of countermeasures, which can be included in the groups that considered it as useful. Most thought that the algorithm test would be a useful method to diagnose CDI but problems such as the health insurance fee, trained personnel, setting and additional purchases of equipment, test referral system, and difficulty in prescription needed improvement. The responses to the plan for implementing the algorithm test were consistent with the method suggested by the existing ESCMID group and IDSA/SHEA guidelines, though it was difficult to clearly distinguish whether to implement them sequentially or simultaneously.
On performing NAAT alone, those that responded that it is the best method and that additional tests are unnecessary were consistent with those that responded that algorithm test is unnecessary. In particular, although the sensitivity is high, whether toxins are produced in patients should be tested via additional toxin AB EIA or CCNA. This is in line with the updated content in the recent IDSA/SHEA guidelines. In 2010 IDSA/SHEA guidelines recommended the NAAT as a stand-alone assay,
11 but in 2018, it recommended including the toxin test, and in particular, institutions without pre-agreed institutional criteria for patient stool submission were recommended to include toxin test in multiple step algorithm rather than NAAT as a stand-alone assay. It is advisable to administer NAAT only in patients with diarrhea symptoms, and the possibility of carrier status in case of a positive result via NAAT should be considered.
6
More than 70% of the institutions conducted surveillance on in-hospital CDI, showing high interest in CDI. Most (93.4%) institutions depended on the CDI assays to determine when to begin quarantining. A positive CDI assay was judged as being tested positive in at least one item, but in case of culture and GDH that cannot distinguish non-toxigenic
C. difficile strains, careful judgment is necessary. Furthermore, there were significantly higher number of people who ended quarantine depending on the disappearance of diarrhea symptoms, but some institutions relied on the CDI assay results to end quarantine. Considering that repeated tests of the CDI assays and tests to determine full recovery were not recommended,
56 ending quarantine based on the CDI assay results may be inappropriate and should be revised.
There are several limitations to this study. Although the number of institutions and their regions were higher than in the 2015 survey, the number of institutions participated are still far below the total number of institutions in Korea. This survey was conducted with laboratory medicine specialists that supervise the tests, but a survey targeting doctors who treat actual patients and prescribe tests is also necessary. And, many laboratories have changed the strategy for CDI diagnosis since 2018. However, this study investigated diagnostic assay methods for CDI in clinical laboratories at the time of 2018, and can be meaningful as a record of the status of the time. Further study is needed on the current status.
This study was conducted after a 2015 nationwide survey on laboratory diagnosis of CDI, which was the first in Korea. Thus, changes since then could be determined, and more questions were added, enabling more diverse aspects to be studied. Among the CDI assays, toxin AB EIA, and NAAT were the most common. Compared to the 2015 survey, the number of institutions using the culture method significantly decreased, and the number of institutions using GDH significantly increased. Most institutions were using a combination of two or more assays, and the combination varied per institution. Through surveying the perception of sensitivity and specificity of each CDI assay alongside the algorithm test, the utility and problems of each assay and algorithm test could be inferred. This study provides useful evidence on the current status of CDI laboratory diagnosis in Korea as well as on items that require improvement and is thought to aid in standardizing and improving the CDI laboratory diagnosis in Korea.
Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download