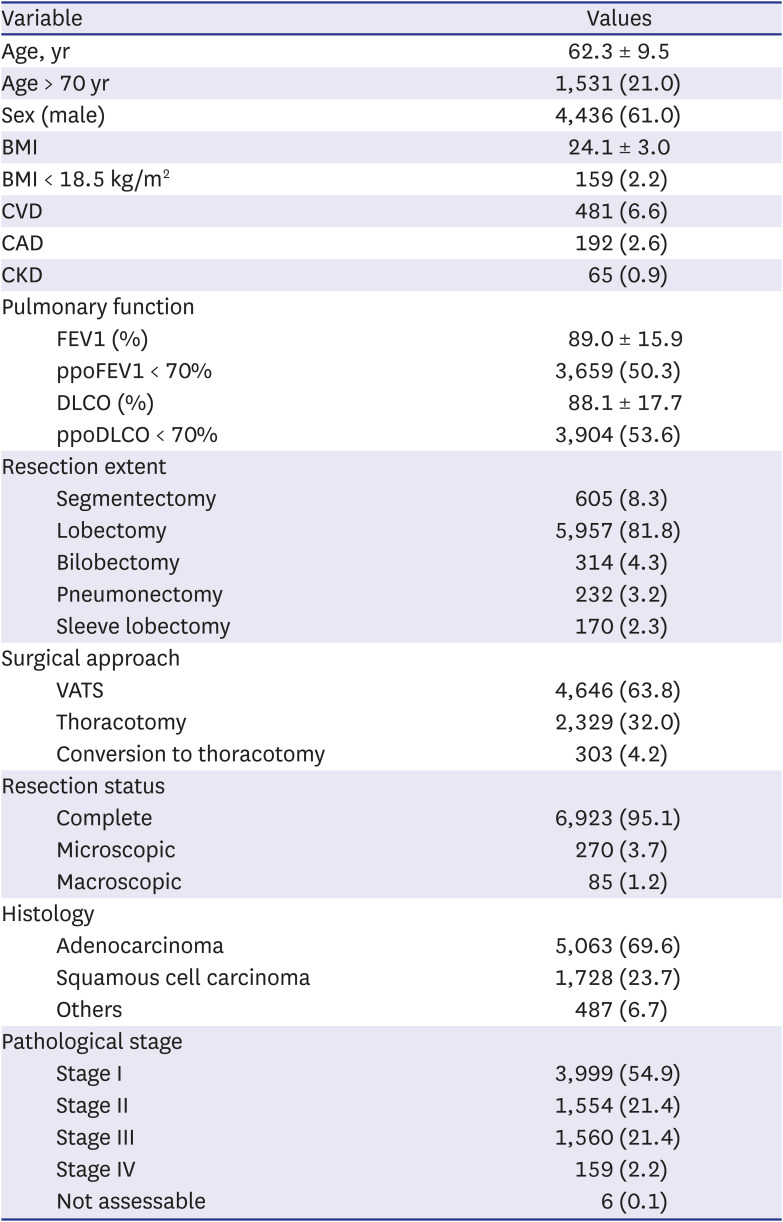
The residual pulmonary function was estimated as follows: it is assumed that the lungs in a normal individual have a total of 19 segments (right upper lobe: 3, right middle lobe: 2, right lower lobe: 5, left upper lobe: 3, lingula: 2, lower left lobe: 4 segments), each contributing equally to ventilation that is 1/19 (nineteenth part).
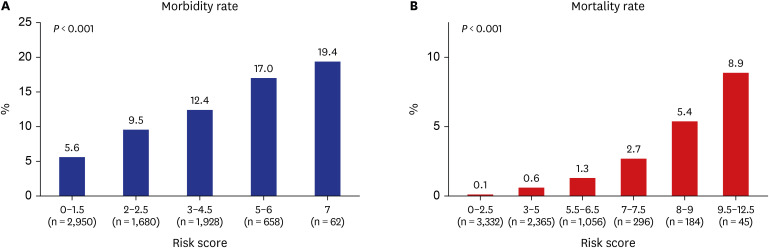
8 Thus, the predicted postoperative forced expiratory volume in 1 second (ppoFEV1) and diffusing capacity of carbon monoxide (DLCO) were calculated as follows: preoperative FEV1 or DLCO × (19 segments – the number of segments to be removed during the surgery) ÷ 19. Cardiopulmonary complications fundamentally complied with the terminology of the ESTS and Society of Thoracic Surgeons, which included the following: prolonged air leak (lasting more than 7 days), airway stenosis, atelectasis, pneumonia, acute respiratory distress syndrome, bronchopleural fistula, pulmonary embolism, pulmonary edema, respiratory failure requiring reintubation, empyema, recurrent laryngeal nerve palsy, phrenic nerve palsy, chylothorax, postoperative bleeding, atrial arrhythmia, myocardial infarction, stroke, and acute renal failure.
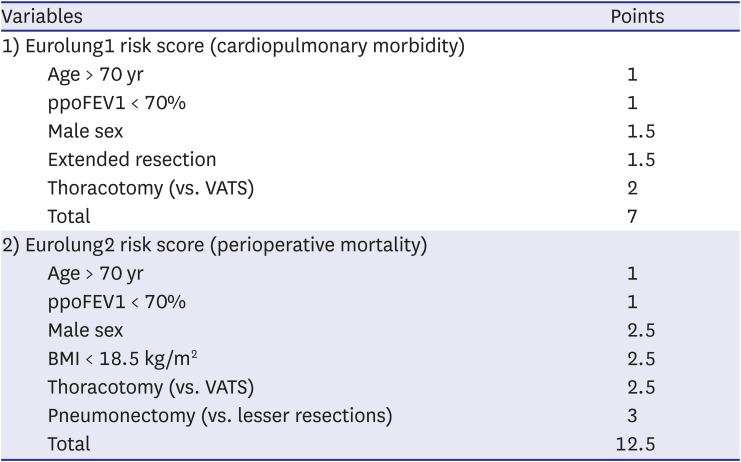
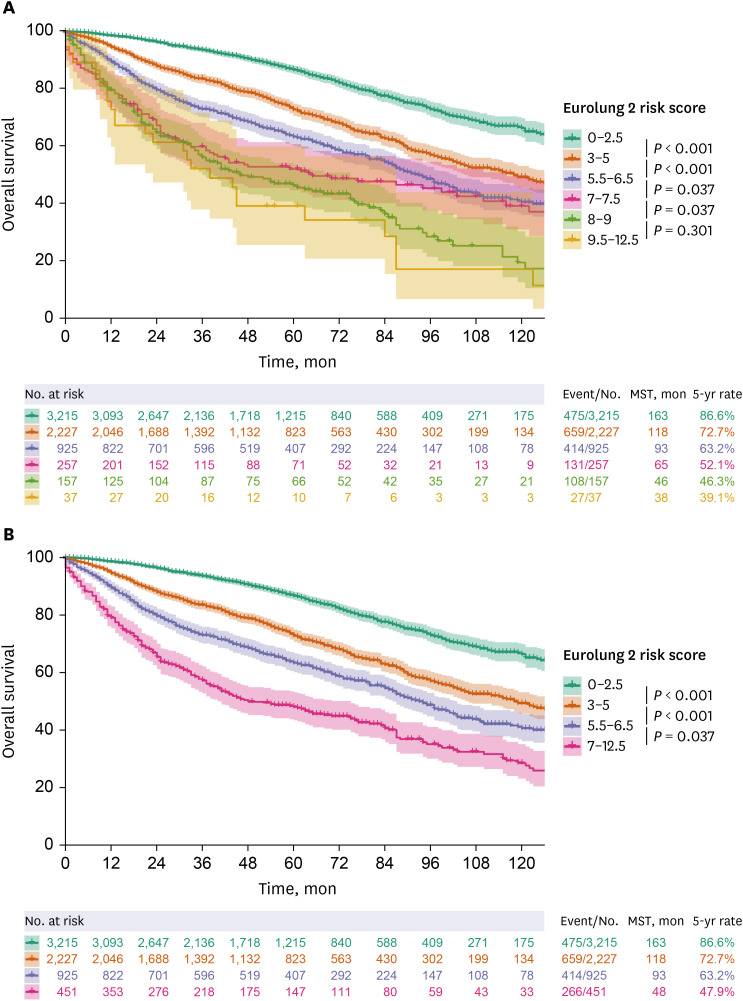
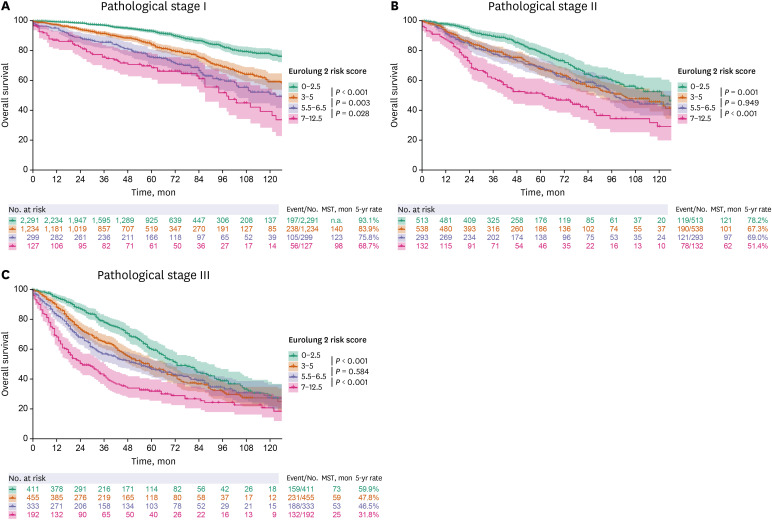
49 Extended resection was defined as follows: 1) chest wall resection, 2) Pancoast tumors, 3) resection of the atrium, superior vena cava, aorta, diaphragm, or vertebra, 4) bronchial sleeve resection, 5) pleuropneumonectomy, 6) sleeve pneumonectomies, or 7) intrapericardial pneumonectomy. Mortality was defined as death within 30 days after surgery or death occurring at any time during the same hospital stay. The overall survival (OS) was calculated as the time interval between the date of surgery and the date of death, which was determined by reviewing the patient records from the Korean National Security Death Index Database. Aggregate Eurolung1 (morbidity) and Eurolung2 (mortality) scoring systems were developed by assigning proportional weightages of the predictors estimates, which are listed in the Eurolung model,
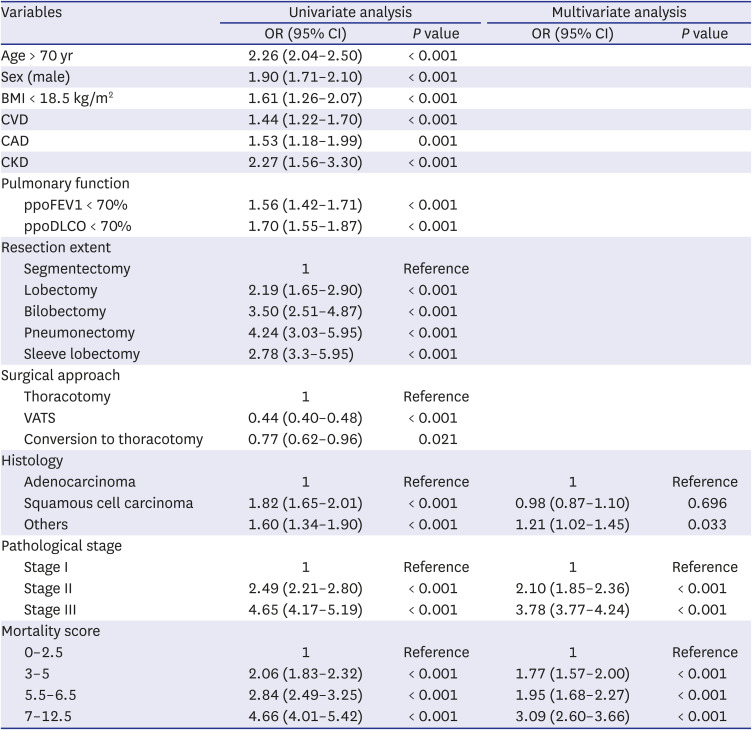
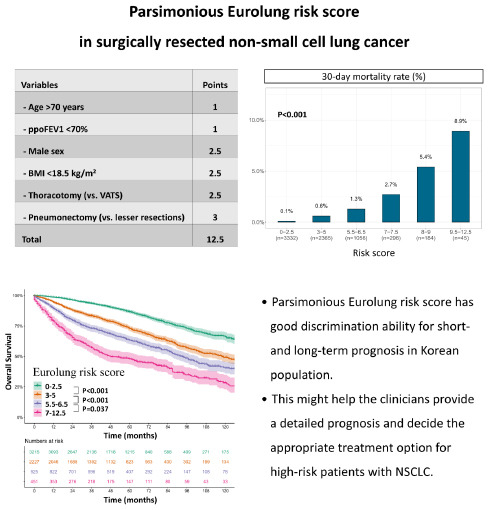
4 assigned a value of 1 to the smallest coefficient. Thus, the Eurolung1 score was calculated as a sum of the points based on the following variables: 1 point for age > 70 years and ppoFEV1 < 70%; 1.5 points for male sex and extended resection; and 2 points for open surgery (as opposed to minimally invasive surgery) (
Table 1). For the Eurolung2 system, age > 70 years and ppoFEV1 < 70% were assigned 1 point; male sex, open surgery (as opposed to minimally invasive surgery), and body mass index (BMI) < 18.5 kg/m
2 were assigned 2.5 points; and pneumonectomy was assigned 3 points (
Table 1).




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download