This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Human immunodeficiency virus (HIV)-associated facial lipoatrophy (FLA) is a stigmatizing side effect associated with the use of highly active antiretroviral therapy. We sought to evaluate the safety and efficacy of the hyaluronic acid filler mixed with micronized cross-linked acellular dermal matrix (HA/MADM) in HIV-associated FLA.
Methods
We conducted an open-label safety and efficacy study in patients with HIV-associated FLA. Fourteen patients received single injection of the HA/MADM, and 13 patients completed the 24-week follow-up evaluation. Treatment efficacy, safety, and patient and physician satisfaction were evaluated. Repeated measure analysis of variance with post-hoc analysis with the Wilcoxon signed rank test was performed to compare and incorporate parameters at each time point.
Results
All 13 patients maintained a significant improvement of the Carruthers Lipoatrophy Severity Scale grade throughout the study period, along with improvement of the depressed volume due to lipoatrophy measured using a three-dimensional camera system. More than 80% of patients and physicians were satisfied with the treatment, and no treatment-related adverse events were reported, except for one case of transient subcutaneous nodule formation.
Conclusion
Our study findings suggest that injectable HA/MADM is a potentially effective and safe treatment option for treating HIV-positive patients with FLA.
Keywords: HIV, Lipoatrophy, Dermal Fillers
INTRODUCTION
Human immunodeficiency virus (HIV)-associated facial lipoatrophy (FLA) is a common skin disease in patients with HIV that is related to the use of highly active antiretroviral therapy (HAART).
1 It is characterized by volume loss of the face that primarily affects the contours of the cheeks, orbits, and temples. HIV-associated FLA can be a social stigma, and it negatively affects patients’ mental health, quality of life, social function, and compliance with HAART.
23
The extended life expectancy of patients with HIV has increased the demand for cosmetic correction of HIV-associated FLA, and filler injections are frequently used for treatment as they improve facial volume. Biodegradable fillers including poly-L-lactic acid (PLLA) and calcium hydroxyapatite (CaHA) are Food and Drug Administration (FDA)-approved for the treatment of HIV-associated FLA, and several studies have been performed to elucidate the effect of other biodegradable and permanent soft tissue fillers.
45 Since existing fillers show various degrees of therapeutic effects and maintenance without standardized guidance, it is necessary to develop a biocompatible, biodegradable filler that is safe and has a long-term volumizing effect. Therefore, the purpose of the present study was to investigate the efficacy and safety of hyaluronic acid filler mixed with micronized cross-linked acellular dermal matrix (HA/MADM) in treating HIV-associated FLA.
METHODS
Study design and population
We conducted a prospective, open-label pilot trial that evaluated the efficacy and safety of HA/MADM filler to treat HIV-associated FLA in 14 patients at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. The study product (MegaNuovo
®, L&C Bio, Inc., Seongnam, Korea) comprises micronized cross-linked acellular dermal matrix and hyaluronic acid (HA) mixed in a weight/volume ratio of 17:1. We included patients with HIV-associated FLA who had a Carruthers Lipoatrophy Severity Scale (CLSS) grade
46 2 or greater for at least one side of the lesion and had not received facial augmentation, lifting, or other treatment of HIV-associated FLA within 2 years.
Treatment and follow-up
All patients were treated with HA/MADM filler and followed up from March 2020 to February 2021. Patients received injections by using the “smile-and-fill” and depot technique to reach the deep subcutaneous level.
6 All injections were performed by the same plastic surgeon (S.Y.S) in an outpatient setting with an optional touch-up at the 4-week follow-up. In the case of bilateral asymmetry, different amounts of filler material were injected for symmetrical correction.
The study duration was 6 months and included five assessment visits: an initial visit at week 0 (baseline) and four follow-up visits at 1, 4, 12, and 24 weeks. A blinded board-certified dermatologist independently evaluated the CLSS grade by comparing digital photographs in a non-chronological order. A three-dimensional skin analysis camera system (Antera 3D
®, Miravex, Dublin, Ireland) was used to perform a quantitative assessment of the depressed volume of lipoatrophy. Additionally, skin elasticity was measured using the Cutometer
® Dual MPA580 (Courage+Khazaka electronic GmbH, Köln, Germany). We assessed the patient and physician satisfaction with treatment outcome using the Global Aesthetic Improvement Scale (GAIS)
7 with five-point scores (1: worse, 2: no change, 3: improved, 4: much improved, and 5: very much improved). Safety assessments included complications assessed through physical examination and self-reporting of related adverse events.
Statistical analysis
Data are presented as numbers (percentages) or means ± SDs. Repeated measures analysis of variance (RM-ANOVA) and subsequent post-hoc analysis with the Wilcoxon signed rank test with the Bonferroni correction was performed to compare and incorporate parameters at each time point. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
Ethics statement
The Institutional Review Board (IRB) of Yonsei University Health System approved this study (IRB No. 1-2019-0066), and informed consent was obtained from each patient.
RESULTS
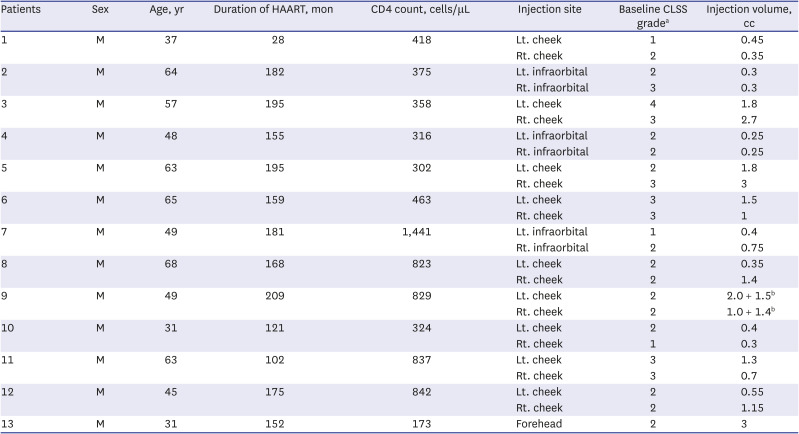
In this prospective pilot study, 14 Korean patients with HIV-associated FLA were enrolled, and 13 completed the 6-month follow-up (one patient was lost to follow-up and retracted his consent). All patients were men, and the mean age at diagnosis was 51.5 ± 12.5 years. The mean duration on HAART was 155.5 ± 46.6 months, the mean CD4 cell count was 577.0 ± 340.4 cells/μL, and 12 (92.3%) patients had < 20 HIV RNA copies/mL. Baseline CLSS grades were evaluated by an independent assessor and were as follows: grade 1, three lesions (12%); grade 2, 14 lesions (56%); grade 3, seven lesions (28%); and grade 4, one lesion (4%). Twelve patients were injected with HA/MADM filler in both cheeks or infraorbital areas, and one patient received an injection in the forehead; thus, 25 sites of injection were noted for further analysis. The mean total volume of HA/MADM used was 1.20 ± 0.99 mL per lesion. Most patients were treated with a single injection of HA/MADM at the initial visit; only one patient received an additional touch-up injection at the 4-week follow up.
Table 1 summarizes the patient characteristics and treatment details.
Table 1
Summary of patient characteristics and treatment details
|
Patients |
Sex |
Age, yr |
Duration of HAART, mon |
CD4 count, cells/μL |
Injection site |
Baseline CLSS gradea
|
Injection volume, cc |
|
1 |
M |
37 |
28 |
418 |
Lt. cheek |
1 |
0.45 |
|
Rt. cheek |
2 |
0.35 |
|
2 |
M |
64 |
182 |
375 |
Lt. infraorbital |
2 |
0.3 |
|
Rt. infraorbital |
3 |
0.3 |
|
3 |
M |
57 |
195 |
358 |
Lt. cheek |
4 |
1.8 |
|
Rt. cheek |
3 |
2.7 |
|
4 |
M |
48 |
155 |
316 |
Lt. infraorbital |
2 |
0.25 |
|
Rt. infraorbital |
2 |
0.25 |
|
5 |
M |
63 |
195 |
302 |
Lt. cheek |
2 |
1.8 |
|
Rt. cheek |
3 |
3 |
|
6 |
M |
65 |
159 |
463 |
Lt. cheek |
3 |
1.5 |
|
Rt. cheek |
3 |
1 |
|
7 |
M |
49 |
181 |
1,441 |
Lt. infraorbital |
1 |
0.4 |
|
Rt. infraorbital |
2 |
0.75 |
|
8 |
M |
68 |
168 |
823 |
Lt. cheek |
2 |
0.35 |
|
Rt. cheek |
2 |
1.4 |
|
9 |
M |
49 |
209 |
829 |
Lt. cheek |
2 |
2.0 + 1.5b
|
|
Rt. cheek |
2 |
1.0 + 1.4b
|
|
10 |
M |
31 |
121 |
324 |
Lt. cheek |
2 |
0.4 |
|
Rt. cheek |
1 |
0.3 |
|
11 |
M |
63 |
102 |
837 |
Lt. cheek |
3 |
1.3 |
|
Rt. cheek |
3 |
0.7 |
|
12 |
M |
45 |
175 |
842 |
Lt. cheek |
2 |
0.55 |
|
Rt. cheek |
2 |
1.15 |
|
13 |
M |
31 |
152 |
173 |
Forehead |
2 |
3 |

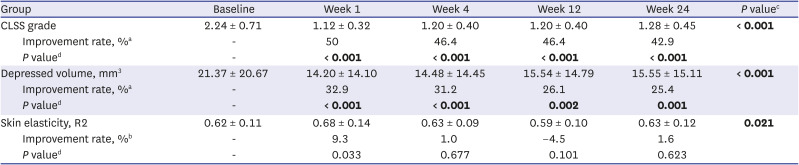
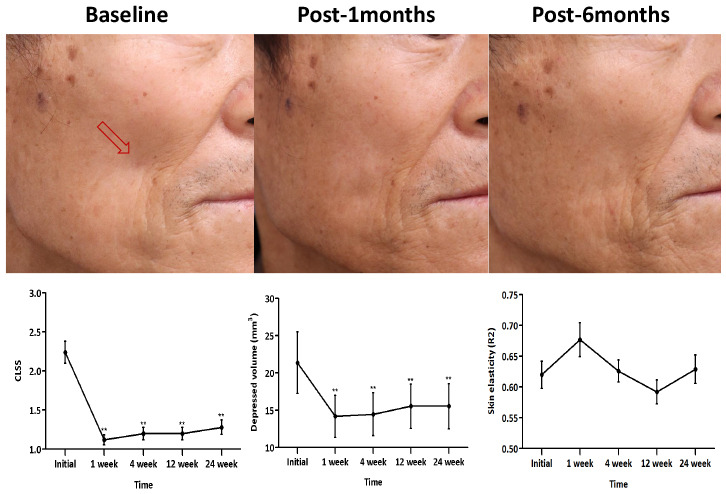
We evaluated the CLSS grade, depressed volume due to lipoatrophy, and skin elasticity of each lipoatrophy lesion from baseline to all time points of follow-up; the findings are summarized in
Table 2. Compared to baseline, the average decreases of the CLSS grade were 1.12 points (50% improvement) after a 1-week follow-up visit and 0.96 points (42.9% improvement) after the 24-week visit. RM-ANOVA and subsequent post-hoc analysis revealed an overall significant improvement of the CLSS grade during the study period along with significant differences in the CLSS grade at all follow-up time points compared with pre-treatment (
Fig. 1A).
Fig. 1
Average (A) CLSS grade, (B) depressed volume measured using the Antera 3D® camera system, and (C) skin elasticity measured using the Cutometer® over the study period. Error bars depict the standard error of the mean.
CLSS = Carruthers Lipoatrophy Severity Scale, R2 = percentage of viscoelasticity.
**P < 0.01 in post-hoc analysis at each time point.
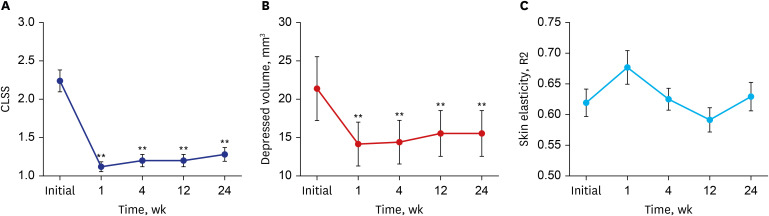

Table 2
Baseline and post-treatment CLSS grade, depressed volume due to lipoatrophy, and skin elasticity
|
Group |
Baseline |
Week 1 |
Week 4 |
Week 12 |
Week 24 |
P valuec
|
|
CLSS grade |
2.24 ± 0.71 |
1.12 ± 0.32 |
1.20 ± 0.40 |
1.20 ± 0.40 |
1.28 ± 0.45 |
< 0.001
|
|
Improvement rate, %a
|
- |
50 |
46.4 |
46.4 |
42.9 |
|
P valued
|
- |
< 0.001
|
< 0.001
|
< 0.001
|
< 0.001
|
|
Depressed volume, mm3
|
21.37 ± 20.67 |
14.20 ± 14.10 |
14.48 ± 14.45 |
15.54 ± 14.79 |
15.55 ± 15.11 |
< 0.001
|
|
Improvement rate, %a
|
- |
32.9 |
31.2 |
26.1 |
25.4 |
|
P valued
|
- |
< 0.001
|
< 0.001
|
0.002
|
0.001
|
|
Skin elasticity, R2 |
0.62 ± 0.11 |
0.68 ± 0.14 |
0.63 ± 0.09 |
0.59 ± 0.10 |
0.63 ± 0.12 |
0.021
|
|
Improvement rate, %b
|
- |
9.3 |
1.0 |
−4.5 |
1.6 |
|
P valued
|
- |
0.033 |
0.677 |
0.101 |
0.623 |

Quantitative skin analysis was performed by measuring the volume of depression and skin elasticity. Using the depression mode of Antera 3D
®, we measured and visualized the depressed volume due to lipoatrophy, which was well correlated with that shown in the clinical photograph (
Figs. 2 and
3). The average depressed volume was 21.37 ± 20.67 mm
3 at the initial visit, and it decreased to 14.20 ± 14.10 mm
3 after the 1-week follow-up visit (32.9% improvement). Similar to the CLSS grade, RM-ANOVA and subsequent post-hoc analysis of the depressed volume showed significant improvement during the overall study period (
Fig. 1B). However, skin elasticity measured with the Cutometer
® showed 9.3% improvement after the 1-week follow-up visit and 1.6% improvement after the 24-week visit. The analysis failed to show statistical significance at all follow-up time points compared with pre-treatment (
Fig. 1C).
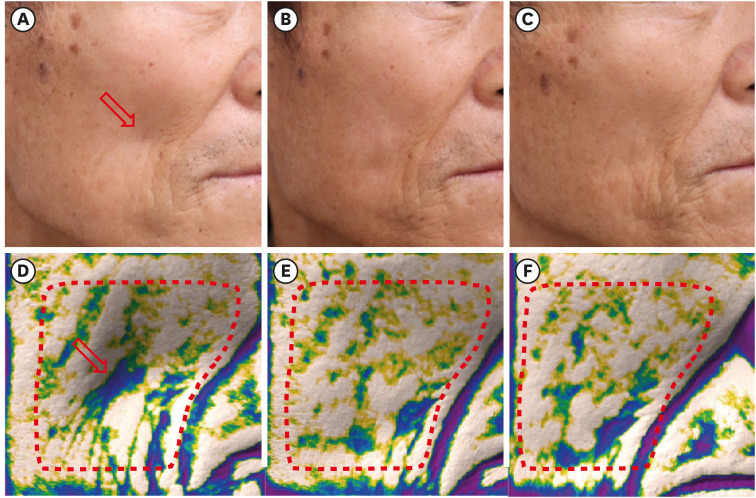
Fig. 2
Clinical photographs of patient number 3 (A) before the injection (baseline), (B) 1 month after the injection, and (C) at the 6-month follow-up visit. Images of the depression mode of the Antera 3D® camera system correspond well with the clinical photographs; (D) before the injection (baseline), (E) 1 month after the injection, and (F) at the 6-month follow-up visit. The depressed volume within the area surrounded by the red dotted line is quantitatively measured. The red arrow indicates the site of filler injection. The figures are published under agreement of the patient.
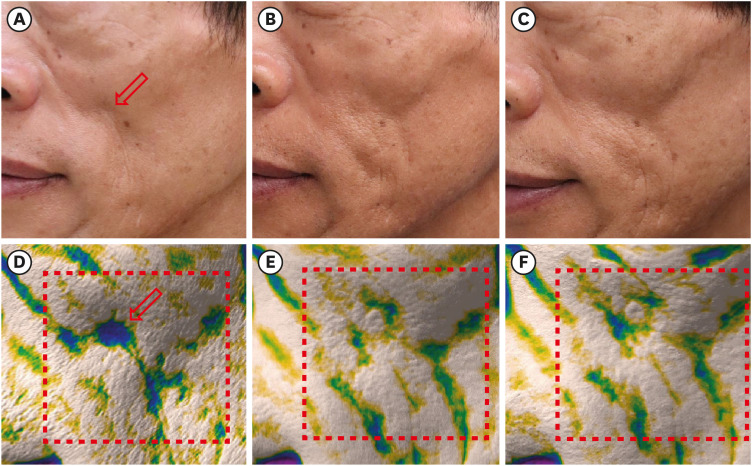

Fig. 3
Clinical photographs of patient number 5 (A) before the injection (baseline), (B) 1 month after the injection, and (C) at the 6-month follow-up visit. Images of the depression mode of the Antera 3D® camera system corresponds well with the clinical photographs; (D) before injection (baseline), (E) after 1-month, and (F) 6-month follow-up visit. The depressed volume within the area surrounded by the red dotted line is quantitatively measured. The red arrow indicates the site of filler injection. The figures are published under agreement of the patient.

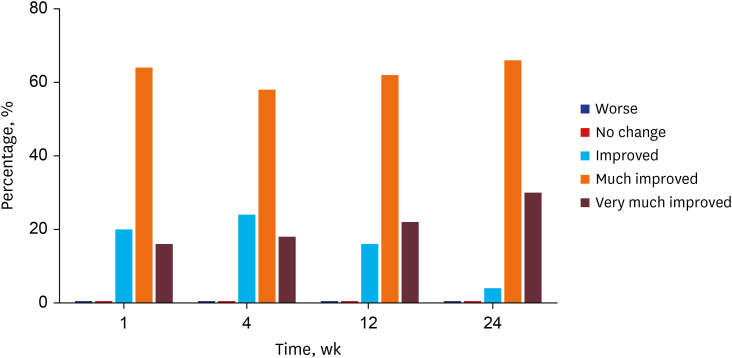
One week after the injection, 80% of patients and physicians rated the treatment outcome as much improved or very much improved based on the GAIS. The degree of satisfaction was gradually increased; almost all (96%) patients and physicians rated the treatment outcome as much improved or very much improved at the 24-week follow-up visit (
Fig. 4). No patients or physicians rated the treatment outcome as no change or worse than before. No permanent or serious treatment-related adverse events were reported during the study period. Only one patient reported a subcutaneous nodule after the 1-week follow-up visit, but it spontaneously resolved by the next visit. None of the patients found exacerbations of HIV-related comorbidities throughout the study period. The mean CD4 cell count measured after the 24-week follow-up visit was unchanged compared to that of the initial visit (542.8 ± 217.4 cells/μL,
P = 0.750).
Fig. 4
Subjective patient and physician satisfaction with treatment outcome assessed by the Global Aesthetic Improvement Scale.

DISCUSSION
In this prospective, open-label pilot study, significant decreases in the CLSS grade and volume of depression were noted with HA/MADM filler in patients with HIV-associated FLA. A single injection of HA/MADM was sufficient to maintain the volumizing effect throughout the study period. Compared to 1 week after the injection, improvement rates of the CLSS grade and depressed volume decreased by only 7.1% and 7.5% at the 24-week follow-up visit, respectively. In addition, the subjective satisfaction of patients and physicians remained high throughout the study period. However, the improvement effect of HA/MADM on skin elasticity was modest (less than 10%) and failed to demonstrate statistical significance. The filler material in the present study was safe; no subject reported any adverse events, except transient subcutaneous nodule formation in one patient.
Patients with HIV who develop persistent lipoatrophy may benefit from plastic surgery, autologous fat transfers, and dermal fillers including PLLA, CaHA, or HA.
48 Among these options, the PLLA filler injection is considered to have the highest efficacy and is relatively safe.
5 However, PLLA requires reconstitution before injection, multiple treatment sessions, and a vigorous manual massage after injection to avoid nodule formation.
49 CaHA was the second FDA-approved filler agent for treating HIV-associated FLA, which may be ideally used deeply over bone for focal enhancement but may not be suitable for correcting volume loss of the midface.
410 HA filler is considered another safe and effective treatment option because of the stability of HA material in vivo and theoretical reversibility with hyaluronidase.
6711 However, HA could result in delayed onset nodules, foreign body granuloma, tissue necrosis due to vessel compression, and allergic reaction.
612
HA/MADM was developed to complement HA filler, which is naturally not replaced by autologous tissue. The manufacturing process of HA/MADM involves cross-linking of the collagen matrix at the level of the peptides and collagen fibers by using electron beams, which gives the filler material augmented tensile strength and a stratified appearance with structural integrity.
131415 HA/MADM contains the extracellular components necessary for cell integration and promotes long-term extracellular matrix remodelling through increased expressions of type I collagen, matrix metalloproteinases-1 (MMP-1), MMP-2, and transforming growth factor-ß for up to 6 months.
13 HA/MADM can also promote adipocyte survival via increased vascular endothelial growth factor expression
14 and showed higher volume retention than the fat graft in a mouse model.
16 Considering the pathogenesis of HIV-associated FLA, including reduced adipogenesis and increased adipocyte apoptosis,
5 HA/MADM may have an advantage over other filler materials by recovering the patient’s own adipose tissue. Limitations of our study include the relatively small sample size, absence of a control group, and lack of female patients. Moreover, this study's relatively short follow-up period limited the assessment of the long-term safety and efficacy of the HA/MADM filler. However, this study used a three-dimensional imaging device to measure volumetric changes and HA/MADM filler retention at each lesion and all time points. This may complement the efficacy evaluation and help assess the durability of this HA/MADM filler.
This study shows that the HA/MADM filler is a safe and effective treatment modality to correct HIV-associated FLA. A single injection of HA/MADM was sufficient to maintain correction for up to 6 months. Future randomized clinical trials with a large sample size and longer study period may confirm its long-term efficacy, durability, and safety. Furthermore, the authors expect that filler material composed of extracellular matrix components of human adipose tissue may exert a more long-term volumizing effect.
ACKNOWLEDGMENTS
The authors would like to thank the L&C Bio Inc. for providing the study product (MegaNuovo®, L&C Bio, Inc., Seongnam, Korea). However, L&C Bio had no role in the study design, implementation, data collection, data analysis, data interpretation, manuscript preparation, manuscript review, or manuscript approval.
References
1. Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000; 14(3):F25–F32. PMID:
10716495.

2. Corless IB, Kirksey KM, Kemppainen J, Nicholas PK, McGibbon C, Davis SM, et al. Lipodystrophy-associated symptoms and medication adherence in HIV/AIDS. AIDS Patient Care STDS. 2005; 19(9):577–586. PMID:
16164384.

3. Rajagopalan R, Laitinen D, Dietz B. Impact of lipoatrophy on quality of life in HIV patients receiving anti-retroviral therapy. AIDS Care. 2008; 20(10):1197–1201. PMID:
18608076.

4. Jagdeo J, Ho D, Lo A, Carruthers A. A systematic review of filler agents for aesthetic treatment of HIV facial lipoatrophy (FLA). J Am Acad Dermatol. 2015; 73(6):1040–1054.e14. PMID:
26481056.

5. Koethe JR, Lagathu C, Lake JE, Domingo P, Calmy A, Falutz J, et al. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020; 6(1):48. PMID:
32555389.

6. Ho D, Jagdeo J. Safety and efficacy of a volumizing hyaluronic acid filler for treatment of HIV-associated facial lipoatrophy. JAMA Dermatol. 2017; 153(1):61–65. PMID:
27806168.

7. Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013; 39(11):1602–1612. PMID:
24093664.

8. Shuck J, Iorio ML, Hung R, Davison SP. Autologous fat grafting and injectable dermal fillers for human immunodeficiency virus-associated facial lipodystrophy: a comparison of safety, efficacy, and long-term treatment outcomes. Plast Reconstr Surg. 2013; 131(3):499–506. PMID:
23142937.
9. Moyle GJ, Brown S, Lysakova L, Barton SE. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006; 7(3):181–185. PMID:
16494632.

10. Carruthers A, Carruthers J. Evaluation of injectable calcium hydroxylapatite for the treatment of facial lipoatrophy associated with human immunodeficiency virus. Dermatol Surg. 2008; 34(11):1486–1499. PMID:
18811715.

11. Skeie L, Bugge H, Negaard A, Bergersen BM. Large particle hyaluronic acid for the treatment of facial lipoatrophy in HIV-positive patients: 3-year follow-up study. HIV Med. 2010; 11(3):170–177. PMID:
19780861.

12. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009; 25(2):86–94. PMID:
19415575.

13. Lee JH, Kim HG, Lee WJ. Characterization and tissue incorporation of cross-linked human acellular dermal matrix. Biomaterials. 2015; 44:195–205. PMID:
25617138.

14. Park TH, Choi WY, Lee JH, Lee WJ. Micronized cross-linked human acellular dermal matrices: an effective scaffold for collagen synthesis and promising material for tissue augmentation. Tissue Eng Regen Med. 2017; 14(5):517–523. PMID:
30603506.

15. Yang CE, Kim SJ, Kim JH, Lee JH, Roh TS, Lee WJ. Usefulness of cross-linked human acellular dermal matrix as an implant for dorsal augmentation in rhinoplasty. Aesthetic Plast Surg. 2018; 42(1):288–294. PMID:
29124378.

16. Kim JH, Kim SE, Kim YJ, Kim YW, Cheon YW. Comparison of volume retention and biocompatibility of acellular dermal matrix/hyaluronic acid filler to autologous fat grafts in a mouse model. Aesthetic Plast Surg. 2020; 44(3):986–992. PMID:
32232518.










 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download