INTRODUCTION
With the emergence of the coronavirus disease 2019 (COVID-19) pandemic in Wuhan, China toward the end of 2019, the U.S. Food and Drug Administration (FDA) authorized two mRNA COVID-19 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) in December 2020, followed by viral vector vaccines ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and Ad26.COV2. S (Johnson and Johnson).
1
The role of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for COVID-19, in the development of systemic rheumatic disease is unknown. COVID-19 can cause hyperinflammation, and multiple studies have reported the development of autoantibodies in COVID-19 patients.
2
Dermatomyositis (DM) is an uncommon inflammatory disorder characterized by various dermatologic changes, proximal muscle weakness, and extra muscular manifestations such as interstitial lung disease (ILD).
3
In this case, a 43-year-old female was diagnosed with DM 10 days following the second dosage of the BNT162b2 mRNA COVID-19 vaccination, in the absence of any additional triggering factors.
Go to :

CASE DESCRIPTION
A 43-year-old Asian Indian female with no significant past medical or travel history with no prior SARS-CoV-2 infection. On May 15, 2021, she received a second dose of BNT162b2 mRNA COVID-19 vaccination at a local center. Approximately 10 days following the vaccination, the patient was presented to the emergency department with an itchy, erythematous rash all over her face, trunk, and hands. She had progressed to an inability to walk, difficulty rising from a chair and climbing stairs, followed by difficulty washing her hair or dressing on her own, and an inability to turn her neck or stretch her hands. On systemic review, we reported polyarthralgia and an unintentional 10 kg weight loss over the 1 month prior to presentation. She had no history of headaches, fever, or dysphagia. She denied any history of recent infections or potential exposure to toxic or medical agents. There was no known family history of autoimmune disease or consanguineous marriage.
On physical examination, she appeared ill with normal vital signs. Her height and weight were 158 cm and 43 kg, respectively. She had a diffuse pruritic rash over her upper limbs and over the dorsum of her hands, along with V-shaped rashes on her neckline (
Fig. 1). There was symmetrical and proximal muscle weakness in both the upper and lower limbs, along with weakness in the neck flexion and normal tone and reflexes. According to the Medical Research Council (MRC) scale, motor strength was 3/5 in the upper limbs and both lower limbs. Tenderness and synovitis were also detected symmetrically in all of the metacarpophalangeal joints, proximal interphalangeal joints, and wrist joints. Otherwise, examination of the abdomen, precordium, respiratory system, and lymph nodes was unremarkable.
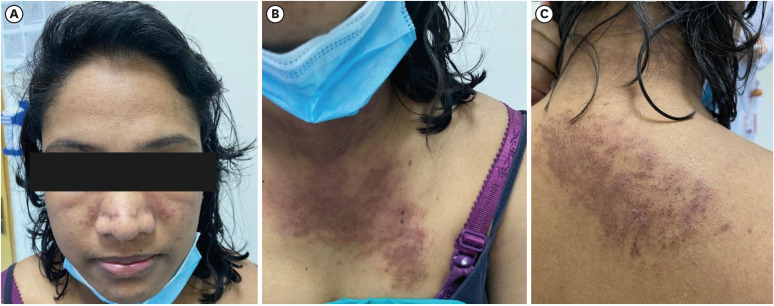 | Fig. 1 Dermatological changes seen in this patient’s face, chest, and back (including a pruritic rash, along with V-shaped rashes on her neckline). Published under informed consent of the patient.
|
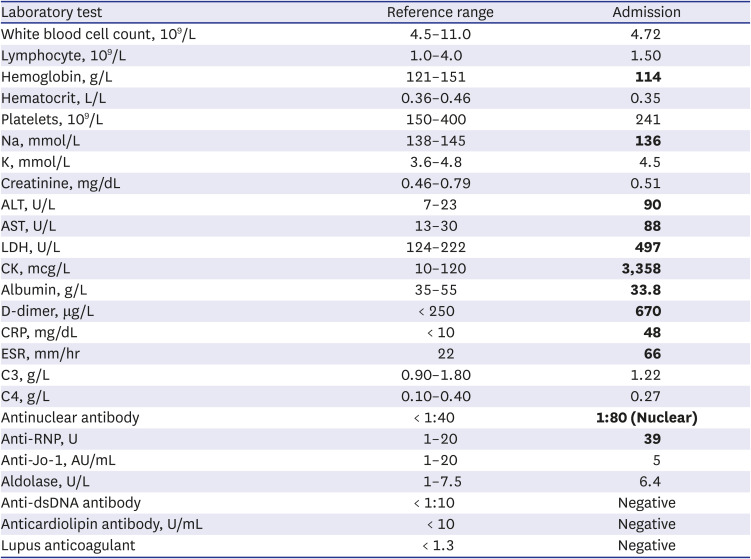
Laboratory studies revealed mild normocytic normochromic anemia, an increase in erythrocyte sedimentation rate of 66 mm/hour (20 mm/hour), a moderate increase in C-reactive protein of 48 mg/L (5 mg/L), an increase in lactate dehydrogenase (LDH) of 497 U/L (222 U/L), and an increase in aspartate aminotransferase (AST) of 88 U/L (< 30 U/L). The alanine aminotransferase (ALT) level was elevated to 90 U/L (23 U/L), while the serum creatine kinase level was markedly elevated to 3,358 mcg/L (120 mcg/L).
Further workup showed weakly positive anti-nuclear antibodies (ANA) with a titer of 1/80 titration (nuclear pattern) and positive anti-ribonucleoprotein (anti-RNP). However, antibodies to Jo-1 or other extractable nuclear antigens were negative. Serum anti-dsDNA, rheumatoid factor, anti-cyclic citrullinated peptides, antiphospholipid antibodies, and antiglobulin test results were all negative (
Table 1). Her complements were normal. Other tests, including thyroid function tests, procalcitonin, and urine analysis, were all within normal limits. All viral panels were negative for SARS-CoV-2, hepatitis B and C viruses, Epstein-Barr virus, herpes simplex virus, coxsackievirus, cytomegalovirus, human immunodeficiency virus, and parvovirus B19.
Table 1
Basic laboratory investigations
|
Laboratory test |
Reference range |
Admission |
|
White blood cell count, 109/L |
4.5–11.0 |
4.72 |
|
Lymphocyte, 109/L |
1.0–4.0 |
1.50 |
|
Hemoglobin, g/L |
121–151 |
114
|
|
Hematocrit, L/L |
0.36–0.46 |
0.35 |
|
Platelets, 109/L |
150–400 |
241 |
|
Na, mmol/L |
138–145 |
136
|
|
K, mmol/L |
3.6–4.8 |
4.5 |
|
Creatinine, mg/dL |
0.46–0.79 |
0.51 |
|
ALT, U/L |
7–23 |
90
|
|
AST, U/L |
13–30 |
88
|
|
LDH, U/L |
124–222 |
497
|
|
CK, mcg/L |
10–120 |
3,358
|
|
Albumin, g/L |
35–55 |
33.8
|
|
D-dimer, μg/L |
< 250 |
670
|
|
CRP, mg/dL |
< 10 |
48
|
|
ESR, mm/hr |
22 |
66
|
|
C3, g/L |
0.90–1.80 |
1.22 |
|
C4, g/L |
0.10–0.40 |
0.27 |
|
Antinuclear antibody |
< 1:40 |
1:80 (Nuclear)
|
|
Anti-RNP, U |
1–20 |
39
|
|
Anti-Jo-1, AU/mL |
1–20 |
5 |
|
Aldolase, U/L |
1–7.5 |
6.4 |
|
Anti-dsDNA antibody |
< 1:10 |
Negative |
|
Anticardiolipin antibody, U/mL |
< 10 |
Negative |
|
Lupus anticoagulant |
< 1.3 |
Negative |

Electromyography of the left quadriceps muscle exhibited fibrillations and small polyphasic motor unit action potentials. Skin biopsy (with immunofluorescence testing) of the rashes over her trunk showed an atrophic epidermis with flattened ridges, a marked vacuolar interface with pigment incontinence, a superficial and deep perivascular and peri adnexal lymphocytic infiltrate with increased mucin, which is consistent with DM (
Fig. 2).
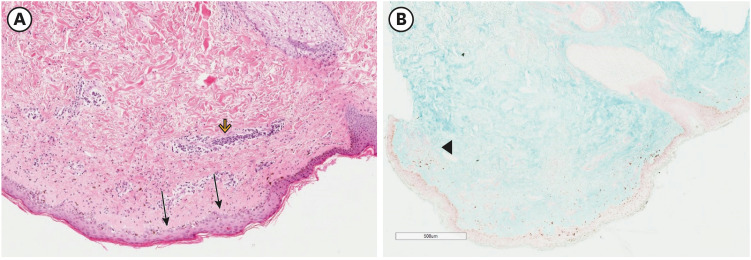 | Fig. 2 Skin biopsy with hematoxylin-eosin and immunofluorescence staining. (A) An atrophic epidermis with flattened ridges (arrows) and a marked vacuolar interface with pigment incontinence (arrowhead). (B) There is a superficial and deep perivascular infiltrate predominantly of lymphocytes with increased amounts of connective-tissue mucin, which is consistent with DM (black arrowhead).
|
A magnetic resonance imaging scan of her thigh muscles showed high signal intensity in T2 fat sat and STIR sequences at the iliopsoas, quadratus femoris, gluteus maximus, obturator externus, and internus, suggesting myositis activity, with no definite muscle atrophy (
Fig. 3). A muscle biopsy was proposed but was declined by the patient. A high-resolution computed tomography of the chest (HRCT) scan showed bilateral basal thick fibrotic bands with patchy ground-glass opacification, all suggestive of an early ILD (
Fig. 4).
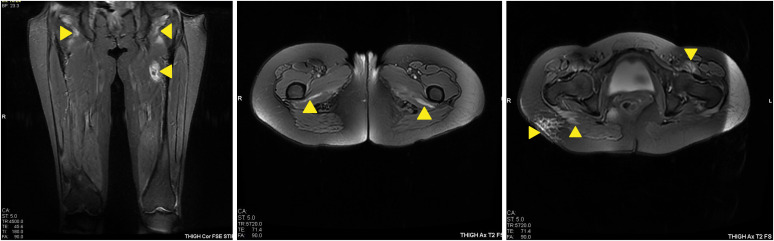 | Fig. 3 Magnetic resonance imaging of the thigh muscles shows areas of myositis (arrows).
|
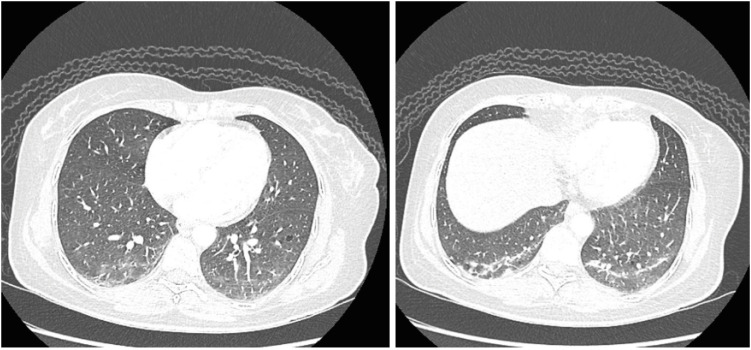 | Fig. 4 High-resolution computed tomography imaging of the chest shows bilateral basal thick fibrotic bands with patchy ground-glass opacification, consistent with early interstitial lung disease.
|
Our patient was diagnosed with DM complicated by ILD following BNT162b2 mRNA COVID-19 vaccination. Her presentation fulfilled Bohan and Peter’s criteria (score of 4/5)
45 as well as the 2017 EULAR/ACR classification criteria (7.7 score).
6 In screening for occult malignancies, CT scans of her abdomen and pelvis, as well as transvaginal ultrasound, were normal. Serum CA-125 levels were normal. Colonoscopy and upper endoscopy revealed no evidence of cancer. The patient was started on prednisolone 60 mg/day, mycophenolate mofetil 1,500 mg/day, and hydroxychloroquine 200 mg/day. After 4 weeks of starting the treatment, she showed a significant improvement in her muscular strength. According to the MRC scale, her motor strength was 4/5 in the upper limbs and both lower limbs. There were no more signs of active arthritis or of an active skin rash. Her investigations showed a modest fall in serum LDH, ALT, and AST levels. The decision was made to keep her on the same drug regimen with physiotherapy. Regular follow up with dermatological, rheumatology, and respiratory clinic has been scheduled.
Ethics statement
Ethics committee approval was received for this study from the ethics committee of Al Azhar University School of Medicine in October 2021 (subject number: MG21BSI053). Informed consent for data collection and publication of the study was obtained from the patient.
Go to :

DISCUSSION
DM is one of the uncommon multi-organ idiopathic inflammatory myopathies. It commonly affects women more than men, with the possibility of onset at any age. Classically, patients present with symmetrical muscle weakness, characteristic skin manifestations, and elevated muscle enzymes.
7
Like other autoimmune conditions, the exact etiology is unknown. However, there are genetic and environmental factors that determine the susceptibility to DM.
8 Several vaccines
9 are among these environmental factors. Researchers believe that vaccine-induced autoimmunity is caused by molecular mimicry between host cell and antigen or directly by vaccine adjuvants, as in autoimmune/inflammatory syndrome induced by adjuvant (ASIA).
10 Examples of vaccines that are thought to have a temporal association with DM include vaccines against hepatitis B, influenza, tetanus toxoid, H1N1, and BCG.
11
mRNA COVID-19 vaccines work by inducing a T-cell-mediated immune response to a protein that has been translated from the mRNA. They have been shown to express a significant level of immunity. It is important to realize that strong induction of type I interferon is a distinguishing feature of mRNA vaccines.
12
Notably, patients with DM have an increase in type I interferon-inducible genes in muscle fibers, endothelial cells, skin, and peripheral blood.
13
COVID-19 vaccines have been linked to several potential side effects. The most prevalent adverse effects were injection site pain, fatigue, headache, myalgia, chills, arthralgia, and fever. However, rare but life-threatening side effects have also been reported. These include, but are not limited to Gullian-Barre syndrome,
14 myocarditis, and pericarditis.
15
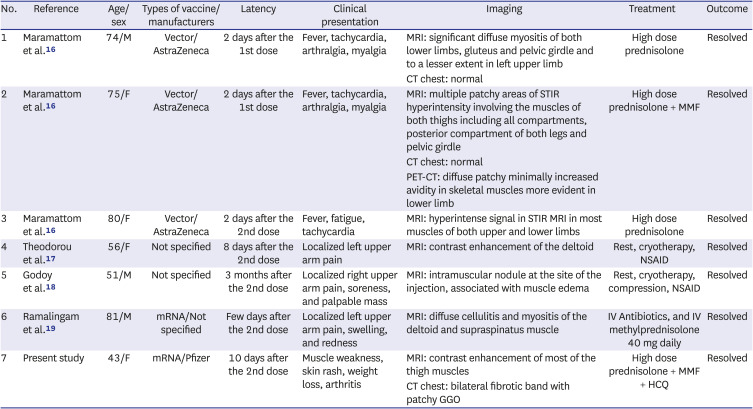
An extensive literature search of PubMed, Scopus, Web of Science, LitCovid, and Google Scholar was performed using the key words "Dermatomyositis," "myositis," "COVID-19," and "vaccine" ranging from December 2020 to December 8, 2021. It has produced six previous reported cases of post-COVD-19 vaccine myositis,
16171819 summarized in
Table 2. In 3 cases, myositis was localized at the same site of injection, while in the 3 other cases following vector vaccines ChAdOx1 nCoV-19, inflammatory myositis affected the proximal muscles of the lower limbs mainly. The latency period between vaccination and the development of symptoms varies, but four out of six cases had symptoms a few days following the second dose of the vaccine, with the age of the affected patients ranging from 51 to 81.
Table 2
Demographic and clinical features of a previously reported myositis following COVID-19 vaccination
|
No. |
Reference |
Age/sex |
Types of vaccine/manufacturers |
Latency |
Clinical presentation |
Imaging |
Treatment |
Outcome |
|
1 |
Maramattom et al.16
|
74/M |
Vector/AstraZeneca |
2 days after the 1st dose |
Fever, tachycardia, arthralgia, myalgia |
MRI: significant diffuse myositis of both lower limbs, gluteus and pelvic girdle and to a lesser extent in left upper limb |
High dose prednisolone |
Resolved |
|
CT chest: normal |
|
2 |
Maramattom et al.16
|
75/F |
Vector/AstraZeneca |
2 days after the 1st dose |
Fever, tachycardia, arthralgia, myalgia |
MRI: multiple patchy areas of STIR hyperintensity involving the muscles of both thighs including all compartments, posterior compartment of both legs and pelvic girdle |
High dose prednisolone + MMF |
Resolved |
|
CT chest: normal |
|
PET-CT: diffuse patchy minimally increased avidity in skeletal muscles more evident in lower limb |
|
3 |
Maramattom et al.16
|
80/F |
Vector/AstraZeneca |
2 days after the 2nd dose |
Fever, fatigue, tachycardia |
MRI: hyperintense signal in STIR MRI in most muscles of both upper and lower limbs |
High dose prednisolone |
Resolved |
|
4 |
Theodorou et al.17
|
56/F |
Not specified |
8 days after the 2nd dose |
Localized left upper arm pain |
MRI: contrast enhancement of the deltoid |
Rest, cryotherapy, NSAID |
Resolved |
|
5 |
Godoy et al.18
|
51/M |
Not specified |
3 months after the 2nd dose |
Localized right upper arm pain, soreness, and palpable mass |
MRI: intramuscular nodule at the site of the injection, associated with muscle edema |
Rest, cryotherapy, compression, NSAID |
Resolved |
|
6 |
Ramalingam et al.19
|
81/M |
mRNA/Not specified |
Few days after the 2nd dose |
Localized left upper arm pain, swelling, and redness |
MRI: diffuse cellulitis and myositis of the deltoid and supraspinatus muscle |
IV Antibiotics, and IV methylprednisolone 40 mg daily |
Resolved |
|
7 |
Present study |
43/F |
mRNA/Pfizer |
10 days after the 2nd dose |
Muscle weakness, skin rash, weight loss, arthritis |
MRI: contrast enhancement of most of the thigh muscles |
High dose prednisolone + MMF + HCQ |
Resolved |
|
CT chest: bilateral fibrotic band with patchy GGO |

In our case, dermatological changes, polymyositis of the upper and lower limbs, and polyarthritis were accompanied by positive serology for ANA and anti-RNP. In addition, the HRCT chest findings were suggestive of DM associated ILD that wasn’t seen in the other 6 cases.
Our patient fulfilled the criteria for ASIA as she had previous exposure to an external stimulus and developed the typical clinical manifestation of autoimmune syndrome. However, this is a very rare phenomenon. For example, in a recent review of the international registry of ASIA that included more than 500 cases from 2011 to 2019, only 2 cases of juvenile DM following the hepatitis B vaccine were reported.
10 Our case may suggest that in patients with genetic predisposition, vaccines may trigger autoimmunity in line with the hypothesis of Talotta et al.
20
To our knowledge, this is the first reported case of classic DM complicated by ILD following COVID-19 vaccination.
We acknowledge that temporal association doesn’t equate to causality. Proof of this causality would require extensive epidemiological studies. A thorough clinical and pathological examination of further patients is required to elucidate this association. Clinicians should be aware of the risk of this unexpected adverse event following COVID-19 vaccination and arrange for appropriate management.
Go to :








 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download