INTRODUCTION
CASE REPORT
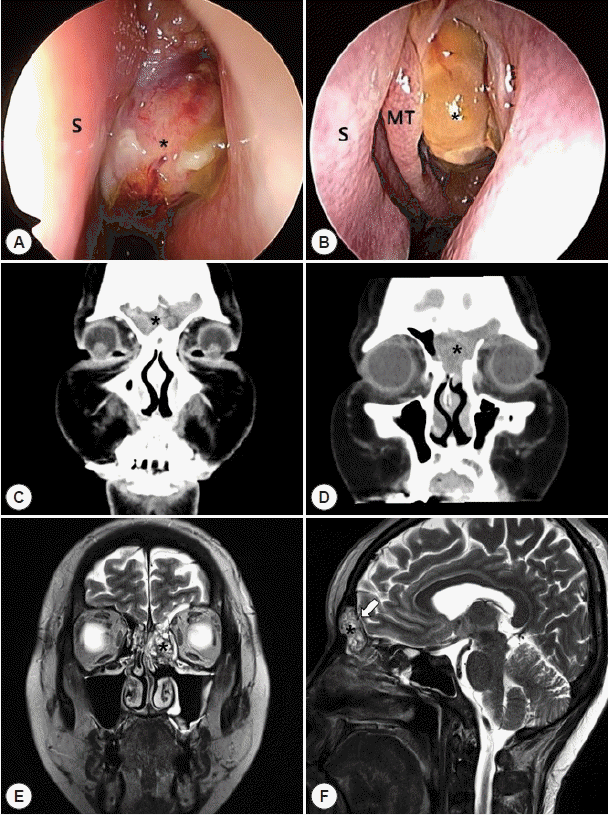 | Fig. 1.Preoperative evaluation of the left ethmoidal sinus tumor. A: Endoscopic view of the left nasal cavity using a 0-degree rigid ENT endoscope during initial physical examination prior to any type of treatment. A huge polypoid mass originating from the left middle meatus (asterisk) can be seen. B: Endoscopic view of the left nasal cavity after 5 cycles of chemotherapy. A significant decrease in tumor size can be noted. The left middle turbinate (MT) and left nasal septum (S) are deviated to the right due to the tumor mass. C: Initial enhanced coronal computed tomography (CT) scan of the left paranasal sinus lesion (asterisk) involving the bilateral ethmoid and frontal sinuses. D: Enhanced coronal computed tomography (CT) scan of the left paranasal sinus lesion (asterisk) after 5 cycles of neoadjuvant chemotherapy demonstrates a significant decrease in tumor size. Additionally, a residual heterogeneously enhancing mass originating from the left ethmoid sinus region and involving the bilateral frontal and ethmoid sinuses can be identified. E: Enhanced magnetic resonance imaging (MRI) scan after 5 cycles of neoadjuvant chemotherapy demonstrates a heterogeneously enhancing T2 high signal intensity lesion (asterisk) as seen on the CT scan. F: Focal thinning of bilateral posterior bony wall of the frontal sinus (white arrow) on the sagittal plane image indicates possible focal bone erosion at the skull base. |
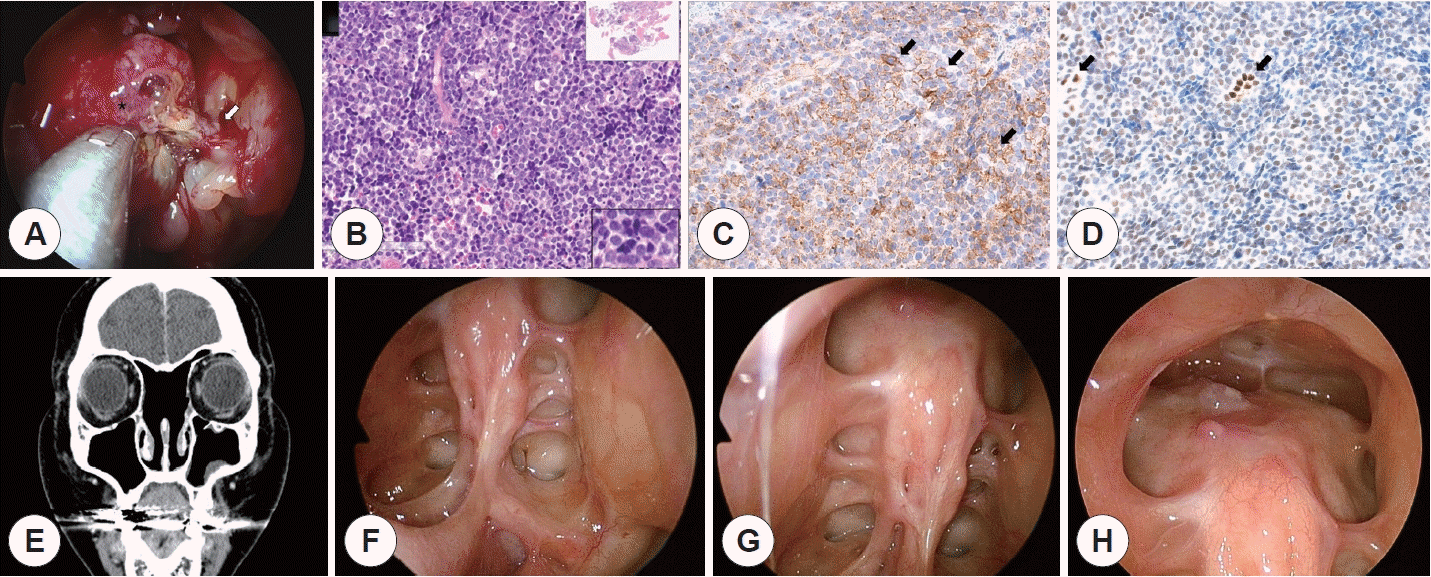 | Fig. 2.Intraoperative view and postoperative pathologic, radiographic and endoscopic findings of left ethmoidal sinus tumor. A: Intraoperative view of the yellow polypoid mass (asterisk) originating from the left ethmoid sinus. The tumor seems to originate from the lateral wall of the left ethmoid sinus. The focal point of origin (white arrow) is marked with a Bovie coagulator for further drilling. B: Microscopic examination of the tumor under hematoxylin and eosin staining shows small round blue cells with a high nuclear-to-cytoplasmic ratio (H-E stain ×200). C: CD-99 positive (black arrows). D: FLI-1 positive (black arrows). E: Coronal image of follow-up enhanced CT scan after 5 cycles of neoadjuvant chemotherapy, complete surgical resection, and adjuvant concurrent chemoradiation therapy (5 cycles of adjuvant chemotherapy and 5,500 cGy of radiation therapy). There is no visible evidence of residual or recurrent disease. F: Endoscopic view of the left post-ethmoidectomy OP bed from the left nasal cavity using a 70-degree rigid ENT endoscope. G: Endoscopic view of the right post-ethmoidectomy OP bed from the right nasal cavity using a 70-degree rigid ENT endoscope. H: Endoscopic view of the post-endoscopic frontal sinusotomy (Draf III) OP bed using a 70-degree rigid ENT endoscope. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download