INTRODUCTION
MATERIALS AND METHODS
Animals
Induction of allergic asthma/administration of LP or BC probiotics
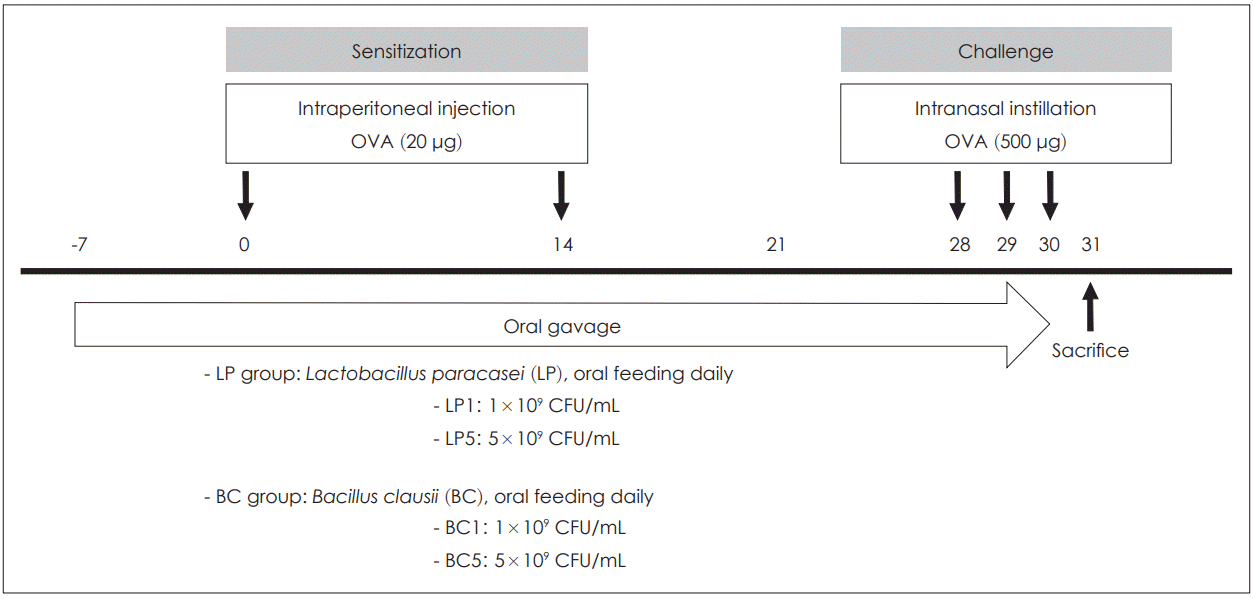 | Fig. 1.Time schedule of the asthma mouse model. Male BALB/c mice were sensitized intraperitoneally to OVA on day 0 and 14 and mice were challenged daily from day 28 until day 30 by intranasal instillation. Mice were treated for 38 days with either LP or BC by oral gavage. Mice in the LP1 or BC1 group were administered 0.2 mL of LP or BC at a dose of 1×109 CFU/mL. Mice in the LP5 or BC5 group were fed 0.2 mL of LP or BC, at a dose of 5×109 CFU/mL, respectively. OVA: ovalbumin, LP: Lactobacillus paracasei, BC: Bacillus clausii. |
Measurement of differential cell counts and titers of Th2 cytokines in bronchoalveolar lavage fluid (BALF)
Histopathology of lung tissue
Measurement of the serum total and OVA-specific IgE
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis
Immunohistochemistry
Statistical analysis
RESULTS
Administration of probiotics reduced allergic pulmonary inflammation
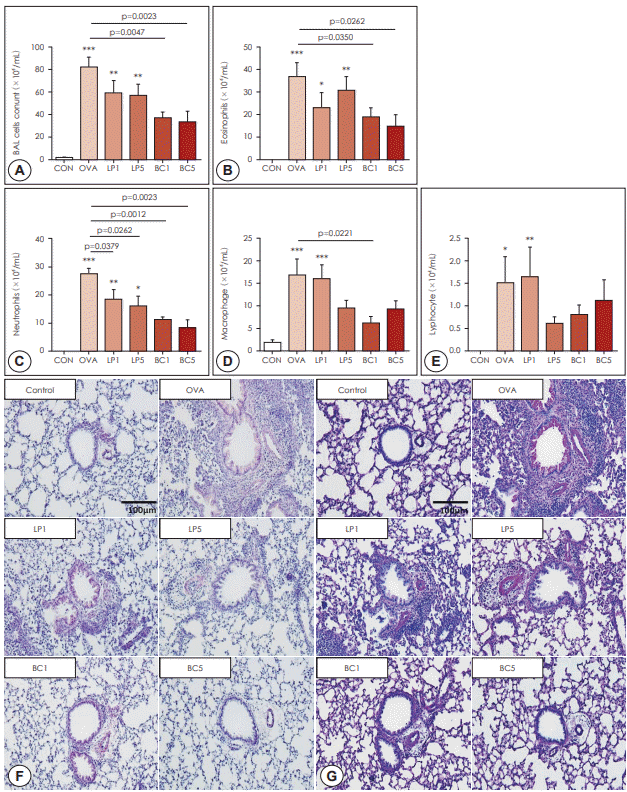 | Fig. 2.Effects of probiotics on hematological and histological changes. BALF was collected 24 h after the last challenge. The lavage fluids were centrifuged, and cell pellets were counted using a hemocytometer after cell staining with trypan blue. A: BALF total cells. B: eosinophils. C: neutrophils, D: macrophages. E: lymphocytes. F: Lung tissues stained with H&E (×200), showing infiltration of inflammatory cells. G: Lung tissues stained with PAS (×200), showing airway goblet cells. The data represent results from seven mice per group. All data are expressed as mean±SD. P-values compared to the OVA group. OVA: ovalbumin. LP: Lactobacillus paracasei, BC: Bacillus clausii. |
Administration of probiotics affected Th2 immune response
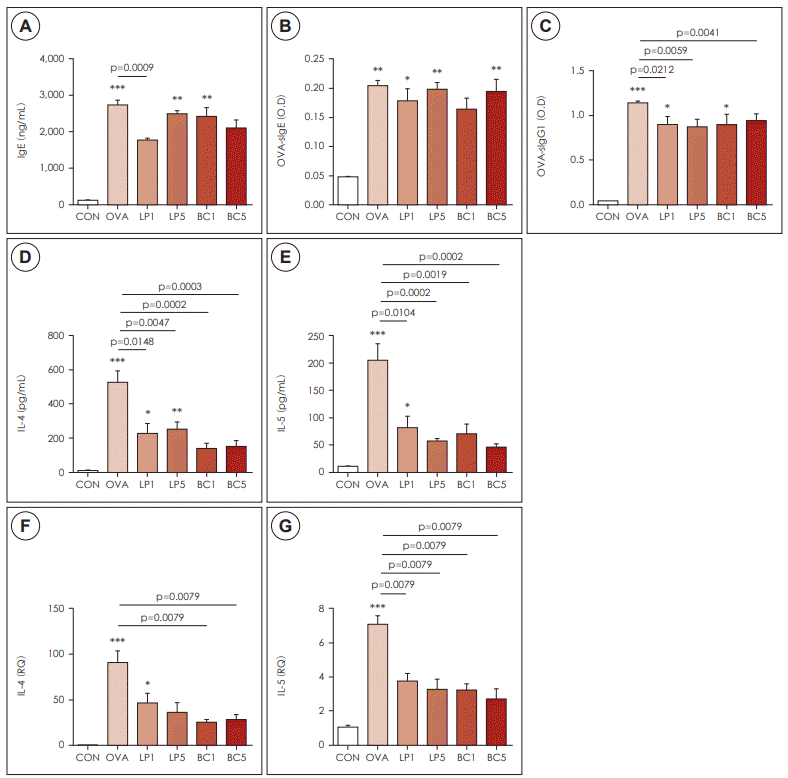 | Fig. 3.Effects of probiotics on Th2 inflammation. A: Total IgE levels in the serum. B: Titers of plasma OVA-specific soluble IgE. C: OVAspecific IgG1 in the six groups. D: IL-4. E: IL-5 concentration in BALF was measured by ELISA. F: Cytokine IL-4. G: IL-5 mRNA levels in
BALF were determined by quantitative RT-PCR. The data represent results from seven mice per group. All data are expressed as mean±SD. P-values compared to the OVA group. OVA: ovalbumin, LP: Lactobacillus paracasei, BC: Bacillus clausii. |
Administration of probiotics suppressed HIF-1α signaling
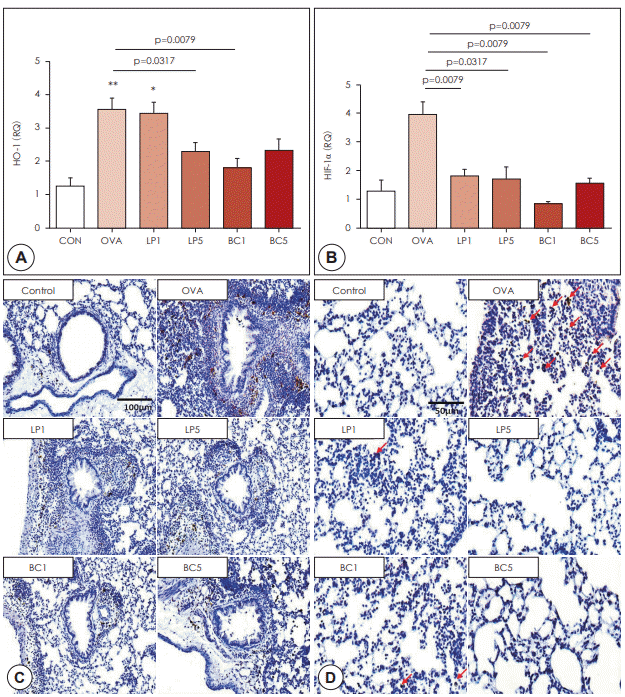 | Fig. 4.Effects of probiotics on hypoxia signaling. A: qRT-PCR was performed to measure the mRNA expression of HO-1 in lung tissue. B: qRT-PCR was performed to measure the mRNA expression of HIF-1α in lung tissue. The data represent results from seven mice per group. C: Immunohistochemical staining of HO-1 in lung tissues (×200 magnification). D: Immunohistochemical staining of HIF-1α in lung tissues (×400 magnification). Red arrows indicate HIF-1α-positive cells. All data are expressed as mean±SD. P-values compared to the OVA group. OVA: ovalbumin, LP: Lactobacillus paracasei. BC: Bacillus clausii. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download