INTRODUCTION
MATERIALS AND METHODS
Chemicals and reagents
Nasal tissues and nasal fibroblast culture
Measurement of cell viability
Detection of histamine type 1 receptor mRNA in nasal fibroblasts
CXCL8 measurements
Western blotting analysis
Immunofluorescent staining
Statistical data analysis
RESULTS
Effects of histamine on cytotoxicity
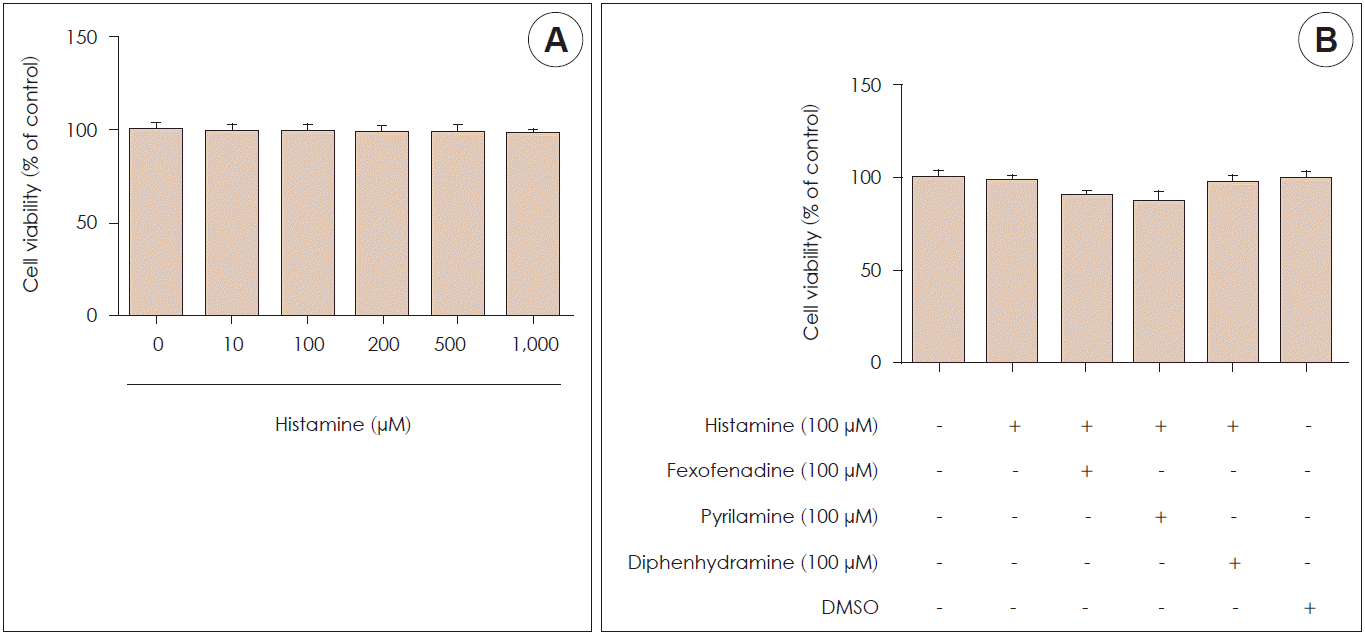 | Fig. 1.Effect of histamine and histamine type 1 receptor antagonists on cell viability in nasal fibroblasts. A: Cytotoxicity tests were performed using 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay at various concentrations of histamine. B: MTT assays were also executed with different mixtures of histamine and each H1R antagonist (fexofenadine, pyrilamine, and diphenhydramine). DMSO: dimethyl sulfoxide. |
Expression of H1R mRNA and effect of histamine on CXCL8 production
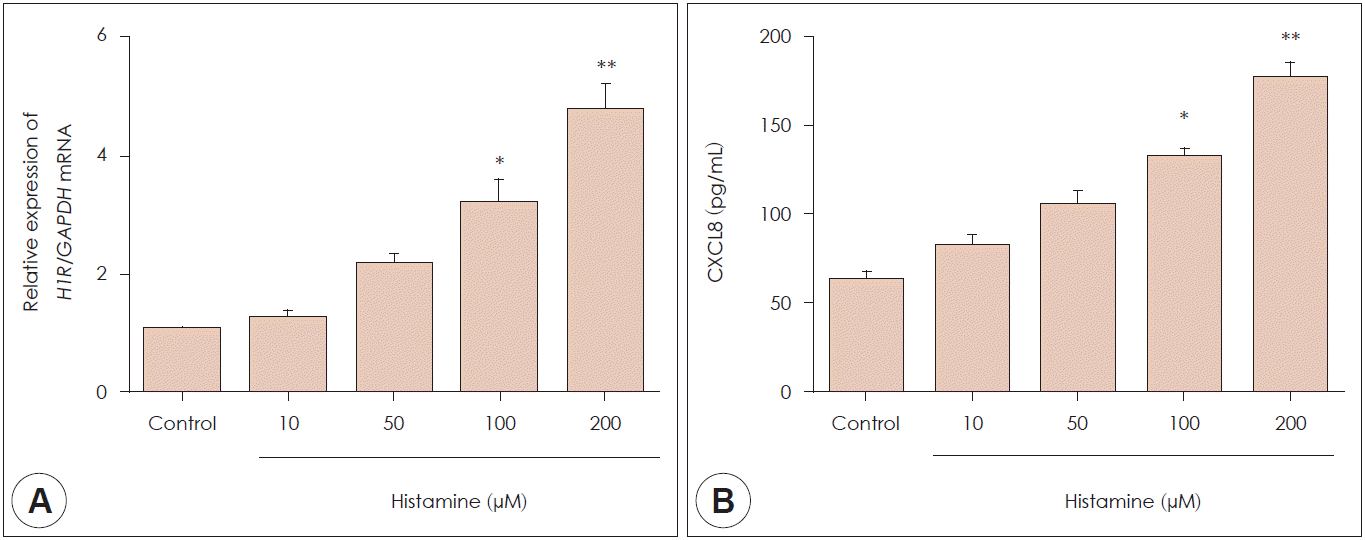 | Fig. 2.Histamine type 1 receptor expression and CXCL8 expression. A: The expression levels of CXCL8 protein in control and nasal polyp tissue were determined using ELISA. B: The expression level of histamine type 1 receptor mRNA was determined by real-time polymerase chain reaction. Values represent mean±SEM. *: p<0.05 vs. control, **: p<0.01 vs. control. |
Effect of H1R antagonists on CXCL8 production of histamine-stimulated nasal fibroblasts
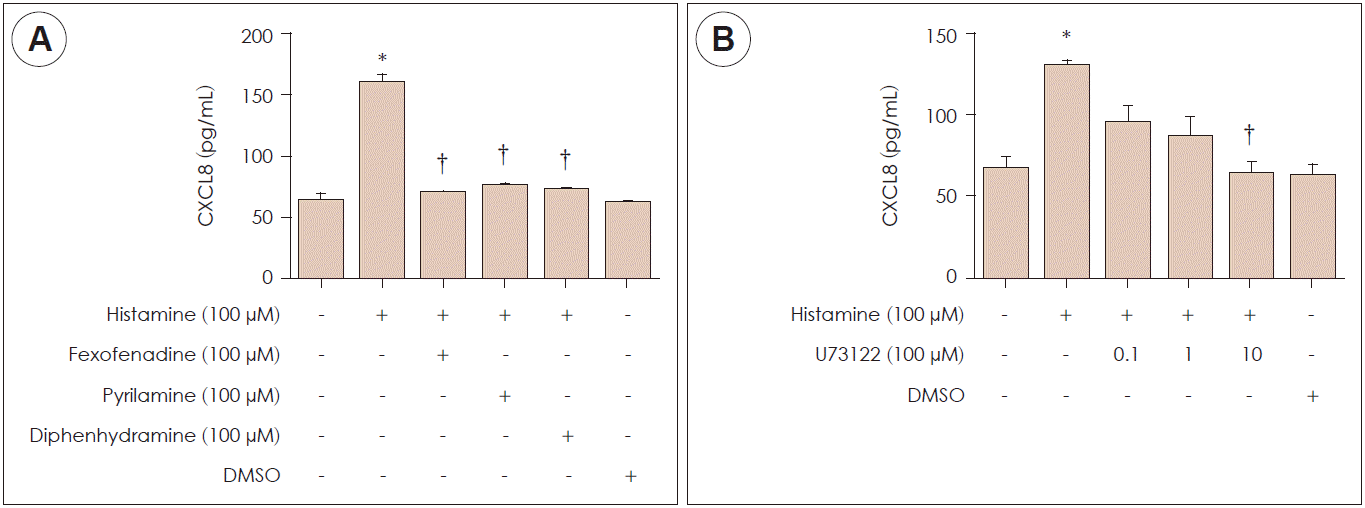 | Fig. 3.Inhibitory effect of histamine type 1 receptor and phospholipase C antagonists on CXCL8 expression in nasal fibroblasts. A, B: The expression levels of CXCL8 protein after treatment with histamine type 1 receptor antagonists (fexofenadine, pyrilamine and diphenhydramine) and phospholipase C inhibitor (U73122) were determined via ELISA. Values represent mean±SEM. *: p<0.05 vs. control, †: p<0.01 vs. histamine alone. DMSO: dimethyl sulfoxide. |
Effect of phospholipase C on histamine-induced CXCL8 production
Effect of histamine on NF-κB activation in histamine-induced CXCL8 production
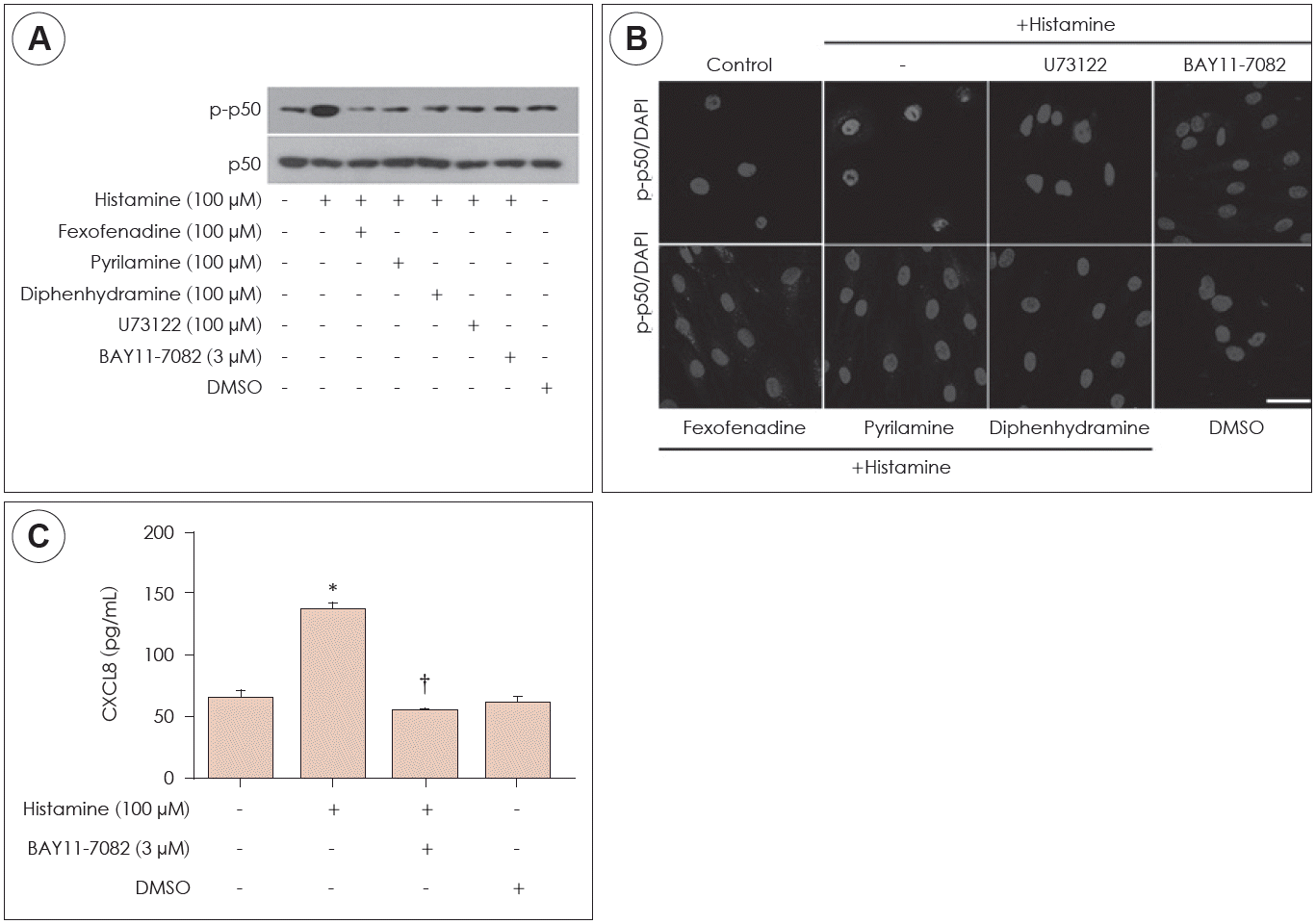 | Fig. 4.Effect of phospholipase C on NF-κB activation in nasal fibroblasts. The expression levels of p-p50 were determined by western blot in nasal fibroblasts (A). Immunolocalization of p-p50 protein was determined immunofluorescent staining (B). The expression level of CXCL8 protein was determined by ELISA (C). Values represent mean±SEM. *: p<0.05 vs. control, †: p<0.01 vs. histamine alone. DMSO: dimethyl sulfoxide; Scale bar=100 μm. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download