1. Chan SL, Chong CCN, Chan AWH, Poon DMC, Chok KS. Management of hepatocellular carcinoma with portal vein tumor thrombosis: review and update at 2016. World J Gastroenterol. 2016; 22:7289–7300.
2. Díaz-González Á, Reig M, Bruix J. Treatment of hepatocellular carcinoma. Dig Dis. 2016; 34:597–602.
3. Moriguchi M, Aramaki T, Nishiofuku H, Sato R, Asakura K, Yamaguchi K, et al. Sorafenib versus hepatic arterial infusion chemotherapy as initial treatment for hepatocellular carcinoma with advanced portal vein tumor thrombosis. Liver Cancer. 2017; 6:275–286.
4. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.
5. Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017; 66:545–551.
6. Colagrande S, Inghilesi AL, Aburas S, Taliani GG, Nardi C, Marra F. Challenges of advanced hepatocellular carcinoma. World J Gastroenterol. 2016; 22:7645–7659.
7. Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020; 107:106–115.
8. Han S, Lee HW, Park JY, Kim SU, Kim DY, Ahn SH, et al. Appraisal of long-term outcomes of liver-directed concurrent chemoradiotherapy for hepatocellular carcinoma with major portal vein invasion. J Hepatocell Carcinoma. 2020; 7:403–412.
9. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018; 68:1429–1440.
10. Sangro B, Gardini AC. Radioembolisation in hepatocellular carcinoma: principles of management. Cross T, Palmer DH, editors. Liver cancers: from mechanisms to management. Cham: Springer International Publishing;2019. p. 139–152.
11. Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, et al. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg. 2005; 140:450–457. discussion 457–458.
12. Hermanek P, Hendson DE, Hutter RVP, Sobin LH. TNM supplement 1993: a commentary on uniform use. Heidelberg: Springer-Verlag;1993. p. 1–142.
13. Edeline J, Crouzet L, Campillo-Gimenez B, Rolland Y, Pracht M, Guillygomarc’h A, et al. Selective internal radiation therapy compared with sorafenib for hepatocellular carcinoma with portal vein thrombosis. Eur J Nucl Med Mol Imaging. 2016; 43:635–643.
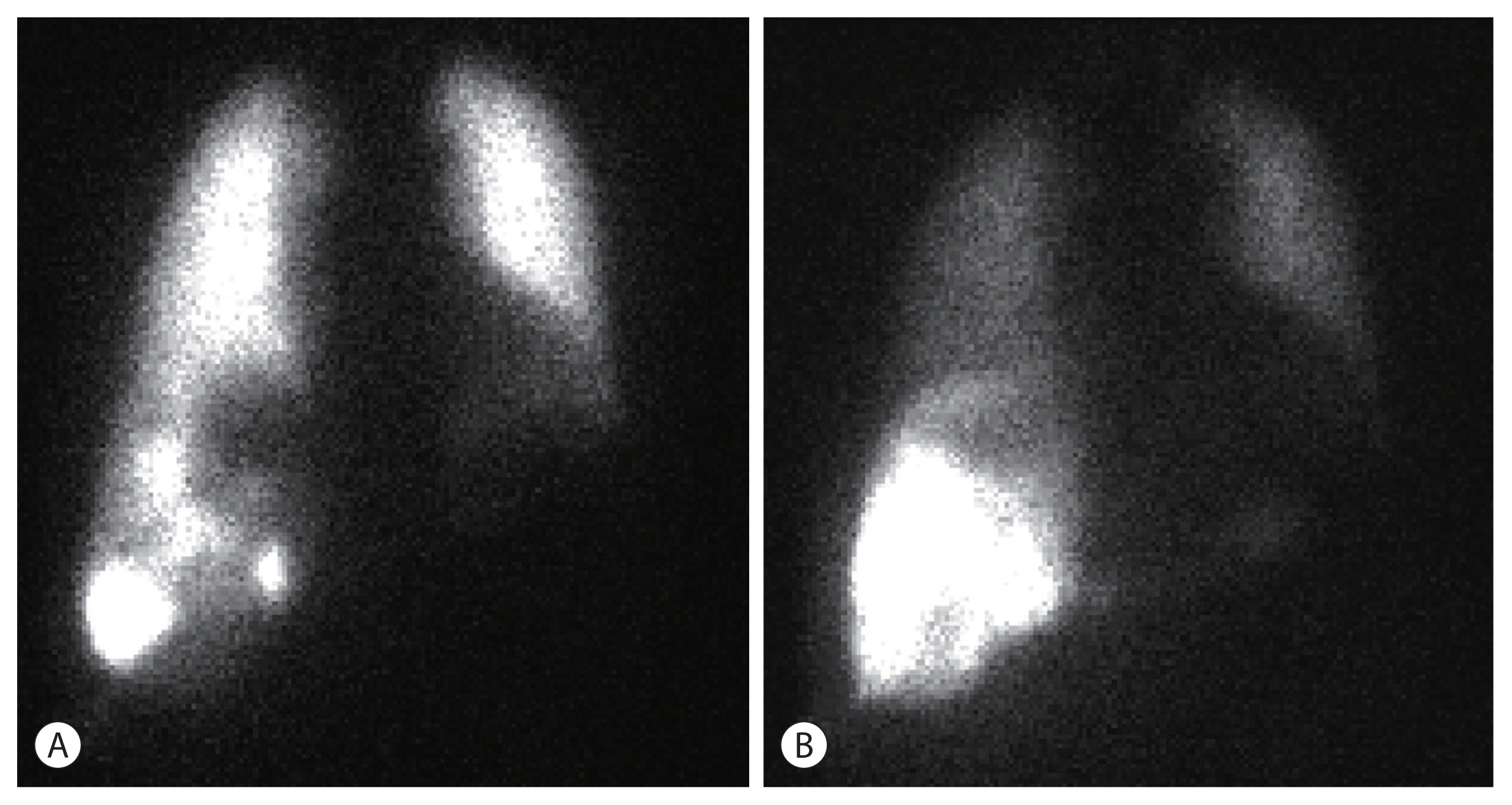
14. Schiro BJ, Amour ES, Harnain C, Gandhi RT. Management of high hepatopulmonary shunts in the setting of Y90 radioembolization. Tech Vasc Interv Radiol. 2019; 22:58–62.
15. Theysohn JM, Schlaak JF, Müller S, Ertle J, Schlosser TW, Bockisch A, et al. Selective internal radiation therapy of hepatocellular carcinoma: potential hepatopulmonary shunt reduction after sorafenib administration. J Vasc Interv Radiol. 2012; 23:949–952.
16. Cha H, Yoon HI, Lee IJ, Koom WS, Han KH, Seong J. Clinical factors related to recurrence after hepatic arterial concurrent chemoradiotherapy for advanced but liver-confined hepatocellular carcinoma. J Radiat Res. 2013; 54:1069–1077.
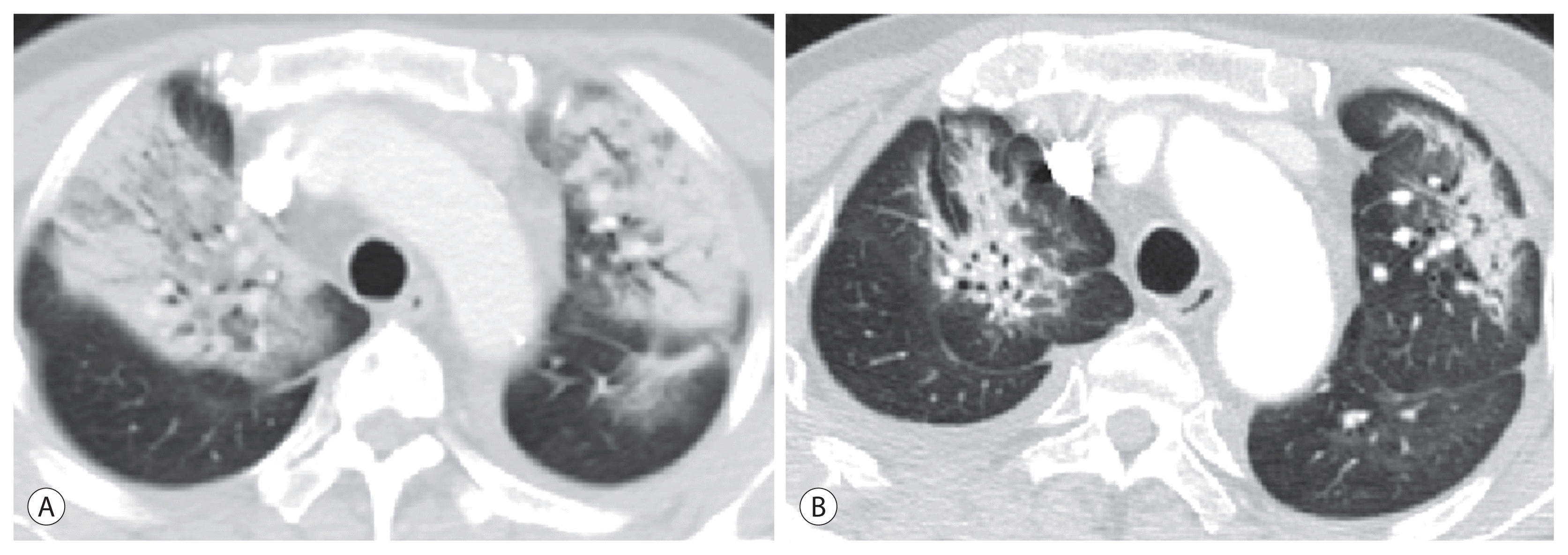
17. Leung TW, Lau WY, Ho SK, Ward SC, Chow JH, Chan MS, et al. Radiation pneumonitis after selective internal radiation treatment with intraarterial 90Yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys. 1995; 33:919–924.
18. Mokdad AA, Singal AG, Yopp AC. Advances in local and systemic therapies for hepatocellular cancer. Curr Oncol Rep. 2016; 18:9.
19. Hendrickson PG, Olson M, Luetkens T, Weston S, Han T, Atanackovic D, et al. The promise of adoptive cellular immunotherapies in hepatocellular carcinoma. Oncoimmunology. 2020; 9:1673129.
20. Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000; 356:802–807.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download