1. Kweon SS. Epidemiology of liver cancer in Korea. J Korean Med Assoc. 2019; 62:416–423.
2. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.
3. Yoon JS, Lee HA, Park JY, Kim BH, Lee IJ, Chon YE, et al. Hepatocellular carcinoma in Korea Between 2008 and 2011: an Analysis of Korean Nationwide Cancer Registry. J Liver Cancer. 2020; 20:41–52.
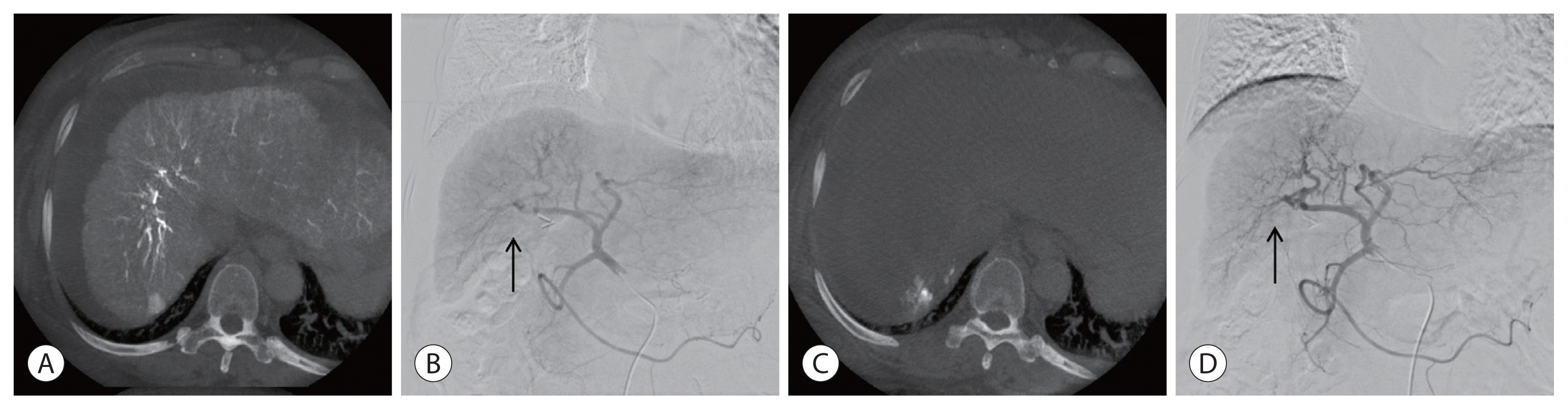
4. Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006; 23:119–125.
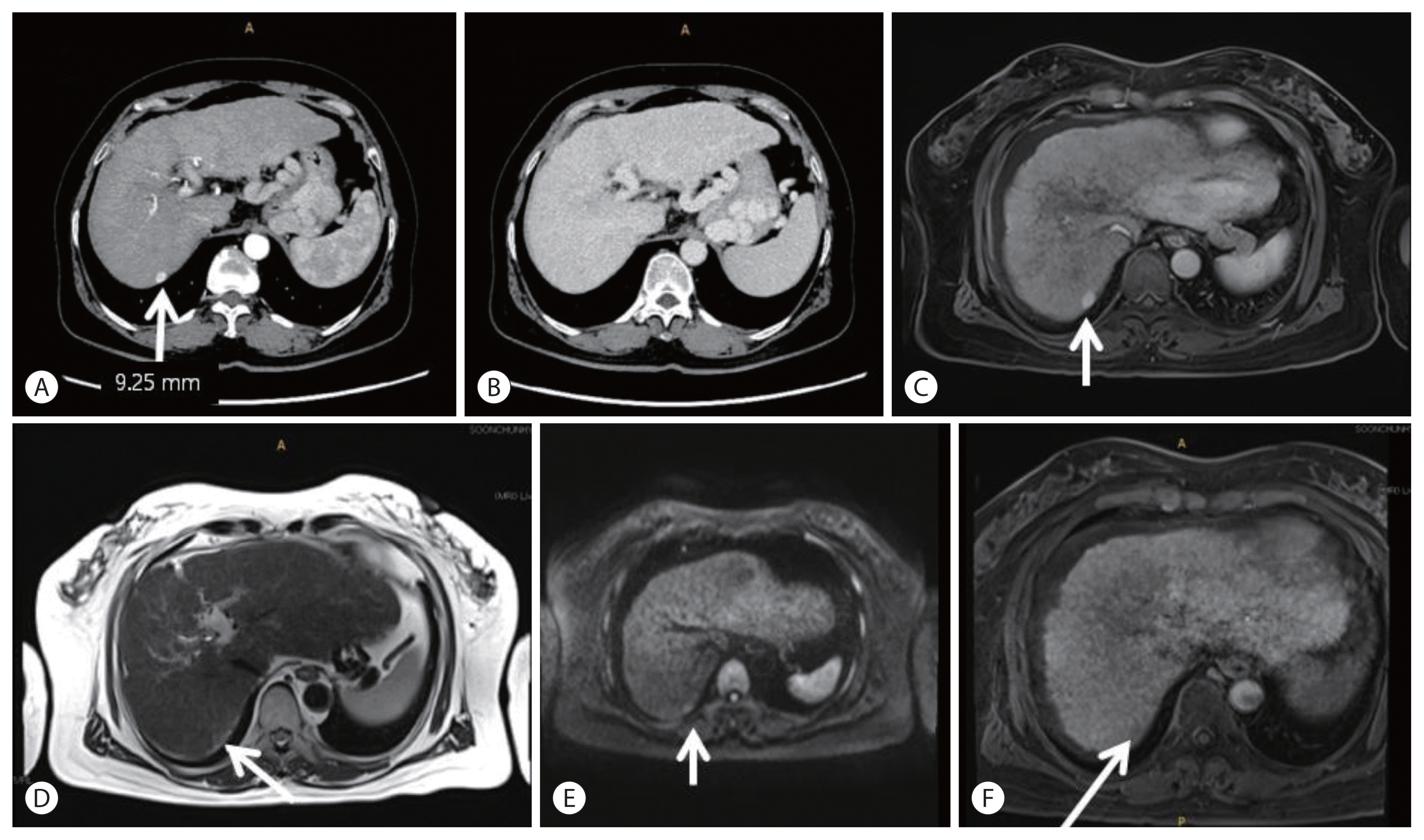
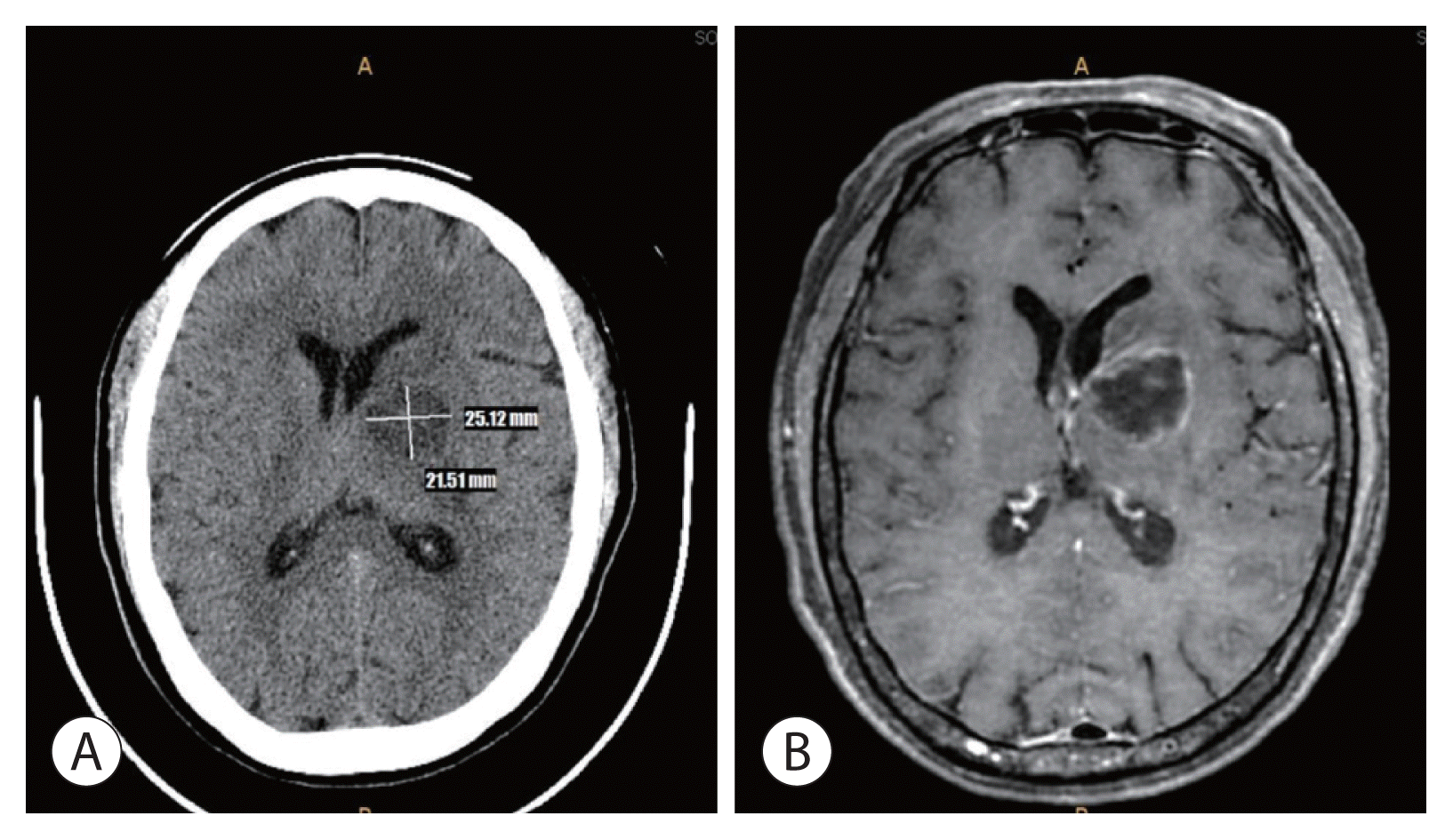
5. Cho TH, Kang SH, Park JY, Kwon TH, Chung YG, Lee HK. Brain abscess after hepatic artery chemoembolization for hepatocellular carcinoma: case report. J Korean Neurosurg Soc. 1998; 27:511–515.
6. Wójcik K, Dalecka-Sztwiertnia E, Piekarska A, Zboińska J, Wrodycki W, Kuydowicz J. Brain abscess: analysis of prevalence and clinical course. Przegl Epidemiol. 2006; 60:265–271.
7. Erdoğan E, Cansever T. Pyogenic brain abscess. Neurosurg Focus. 2008; 24:E2.
8. Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis. 2012; 12:332.
9. Ong CT, Tsai CF, Wong YS, Chen SC. Epidemiology of brain abscess in Taiwan: a 14-year population-based cohort study. PLoS One. 2017; 12:e0176705.
10. Muzumdar D, Jhawar S, Goel A. Brain abscess: an overview. Int J Surg. 2011; 9:136–144.
11. Tarazov PG, Polysalov VN, Prozorovskij KV, Grishchenkova IV, Rozengauz EV. Ischemic complications of transcatheter arterial chemoembolization in liver malignancies. Acta Radiol. 2000; 41:156–160.
12. Cubiella J, Sans M, Llovet JM, Bustamante J, Ferrer A, Caballeria J, et al. Pulmonary abscess as a complication of transarterial embolization of multinodular hepatocellular carcinoma. Am J Gastroenterol. 1997; 92:1942–1943.
13. Wong E, Khardori N, Carrasco CH, Wallace S, Patt Y, Bodey GP. Infectious complications of hepatic artery catheterization procedures in patients with cancer. Rev Infect Dis. 1991; 13:583–586.
14. Lee YJ, Kim GH, Park DY, Kim S, Park CJ, Kim TK, et al. A case of epidural abscess occurred after liver abscess complicated by transarterial chemoembolization in a patient with metastatic cancer to liver. Korean J Gastroenterol. 2013; 61:225–229.
15. Fang XY, Hu LH, Hang YP, Chen YH, Xu XH, Hu XY, et al. Polymicrobial bacteremia after treatment of transcatheter arterial chemoembolization: a case report. Medicine. 2019; 98:e17393.
16. Watchmaker JM, Lipnik AJ, Fritsche MR, Baker JC, Mouli SK, Geevarghese S, et al. Are prophylactic antibiotics necessary prior to transarterial chemoembolization for hepatocellular carcinoma in patients with native biliary anatomy? J Surg Oncol. 2018; 117:1312–1317.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download