Abstract
Primary signet ring neuroendocrine tumors of the liver are extremely rare tumors. Morphologically, they mimic signet ring cell adenocarcinomas; however, the absence of mucin by special stains and the expression of neuroendocrine markers help to diagnose these tumors. We herein report a case of a 47-year-old female who presented with multiple solid and cystic lesions in both liver lobes, which were initially suggested to be biliary cystadenocarcinoma on imaging. Liver biopsy of the lesion revealed the presence of a signet ring neoplasm with diffuse expression of synaptophysin and pan-cytokeratin. The case was subsequently diagnosed as a primary hepatic signet ring neuroendocrine tumor. The patient was offered 3 cycles of chemotherapy and is well preserved after 14 months of diagnosis. Although this is an extremely rare entity, its possibility should be considered in the differential diagnosis of neoplasms characterized by signet ring cell morphology.
Hepatic involvement by neuroendocrine tumors (NETs) is usually seen in metastatic diseases, and primary hepatic NETs are extremely rare, accounting for 0.4% of resected hepatic tumors.1 Primary hepatic NETs with signet ring cell morphology are even more of a rarity, with only 5 cases reported in the English literature. These tumors pose important diagnostic dilemmas as they morphologically resemble various neoplasms, such as metastatic signet ring cell carcinomas, malignant melanoma, plasmacytoma, etc. While immunohistochemical workup is helpful to differentiate between these entities, and it is important to suspect the possibility of neuroendocrine differentiation on histology. We herein report a case of a primary hepatic signet ring NET in a 47-year-old female patient, and review the histological characteristics, immunophenotype and the differential diagnosis of this extremely rare entity. This case report is described according to the CARE guidelines available from https://www.care-statement.org/.
A 47-year-old female presented with complaints of dull aching pain over the right hypochondrium for the last 2 years and shortness of breath for the last 2 months. The pain was intermittent, mild to moderate, and was relieved by oral analgesics. She had a history of vomiting for 2–3 months; the vomiting was projectile, non-bilious, foul-smelling and copious. There was no other significant history of jaundice or gastrointestinal bleeding. She had no prior medical or surgical history, and she had no history of alcohol intake.
On palpation, the liver was palpable 10 cm below the right costal margin, and was tender, smooth and moving with respiration. There was no splenomegaly. The laboratory findings were as follows: white blood cell count, 6,500/μL; hemoglobin 8.5 g/dL, platelets, 135,000/μL; erythrocyte sedimentation rate 18 mm/hr, blood urea nitrogen, 35 mg/dL; serum creatinine, 1.2 mg/dL; serum calcium 8.4 mg/dL; blood glucose 72.3 mg/dL; total bilirubin 0.91 mg/dL; aspartate aminotransferase 26 IU/L; alanine aminotransferase 21 IU/L; alkaline phosphatase 794 IU/L; total protein 7.0 mg/dL; albumin 3.54 g/dL, and prothrombin time-international normalized ratio 1.71. Viral markers, including hepatitis B surface antigen and antibodies to hepatitis C virus, and human immunodeficiency virus were negative. Alpha-fetoprotein level was <5 ng/mL, and carcinoembryonic antigen was 2.0 μg/L. Echocardiography showed left ventricular ejection fraction of 60% and mitral valve prolapse.
On contrast-enhanced computed tomography (CT), the liver was grossly enlarged, with a span of 30.7 cm. There were multiple cystic lesions in both lobes of the liver with peripheral solid component and heterogeneous enhancement (Fig. 1). On venous phase, the solid components remained heterogeneous. Some of the solid components showed calcification.
Positron emission tomography (PET) CT scan demonstrated multiple peripherally fluorodeoxyglucose (FDG)-avid variable-sized cystic lesions in both lobes of the liver, the largest one measuring 10.2×8.8 cm (SUVmax-7.0) (Fig. 2). The cystic lesions showed both complete and incomplete enhancing septa. A few of the masses were partially exophytic, indenting the abdominal wall, and abutting the pancreatic body, stomach, duodenum and right kidney. The portal vein was uninvolved. In addition, multiple FDG-avid variable-sized lymph nodes were seen in the periportal, paraaortic, peripancreatic, aortocaval, right upper paratracheal, subcarinal and pretracheal areas, largest measuring 30×23 mm (SUV max-8.4, periportal lymph node). A few of these lymph nodes showed specks of calcification. The possibility of biliary cystadenocarcinoma was entertained based on these imaging findings.
A percutaneous liver biopsy was obtained from the mass lesion. The biopsy tissue revealed tumor cells arranged predominantly in papillary architecture and in sheets separated by delicate microvasculature. The tumor cells had eosinophilic cytoplasm and contained eccentrically located prominent intracytoplasmic vacuoles, resembling signet ring cells in adenocarcinoma (Fig. 3). The chromatin was granular and nucleoli were inconspicuous. There was no evidence of mucin in the intracytoplasmic vacuoles, by mucin stains (mucicarmine, Alcian blue and periodic acid-Schiff stain). The neoplastic cells were positive for pan-cytokeratin (CK), diffusely positive for synaptophysin and CD56 and weakly positive for chromogranin A (Fig. 4). The tumor cells were negative for CDX-2, TTF-1, CK19, PAX-8, GATA3, and CK20. Ki-67 labeling index was 2–3%. Thus, the case was finally diagnosed as a primary hepatic signet ring NET, grade 1, with multiple lymph node metastasis.
The patient underwent three cycles of chemotherapy with carboplatin, doxorubicin, etoposide and filgrastim, and is currently alive without evidence of disease progression at 14 months of follow-up.
NETs of the liver are usually metastatic; primary hepatic NETs are very rare, and the possibility of a metastatic lesion has to be excluded with extensive workup before a diagnosis of primary hepatic NET can be rendered. First described by Edmondson in 1958, primary hepatic NET has been shown to represent about 0.3% of all NETs, and the mean age at diagnosis is 50 years.1,2 The postulated origin of primary hepatic NET is the neuroendocrine argentaffin cells located within the intrahepatic biliary tree.3
Morphologically, NETs demonstrate a wide variety of appearances; while the majority demonstrate the classic trabecular, nested, ribbon-like pattern, there are rarer variants of NETs, such as lipid-rich, oncocytic, pleomorphic, rhabdoid, clear cell, plasmacytoid and signet ring cell variants.4 Of the variants, signet ring cell variants are very rare, and signet ring cell NETs arising in the stomach, pancreas and lung etc. have been described in sporadic case reports.5 These morphologically resemble signet ring cell carcinomas, and are characterized by the presence of intracytoplasmic vacuoles that push the nucleus to one side of the cell. The intracytoplasmic vacuoles represent aggregates of intermediate filament on electron microscopy.5 The origin of these vacuoles is uncertain, and a degenerative process has been postulated as a mechanism. 6
The presence of a signet ring morphology in a hepatic NET is an extremely rare finding, and only 7 cases of such tumors (5 primary and 2 metastatic) have been published in the English literature to date (Table 1).6–11 Histologically, all cases demonstrated prominent signet ring morphology with intracytoplasmic vacuoles which were negative on mucicarmine and periodic acid-Schiff stain and positive for pan-CK. Two cases were considered to be hepatic metastases from other primaries (rectal and duodenal),9,12 while the remaining 5 cases were considered to be primary hepatic signet ring NETs.6–8,10,11
The identification of the signet ring morphology raises a number of differential diagnoses including metastatic signet ring carcinoma from the gastrointestinal tract, cholangiocarcinoma with signet ring morphology, melanomas, germ cell tumors, plasmacytomas, and NETs. A panel of immunohistochemical markers comprising CDX-2, CK7, CK20, PSA, HepPar-1, Arginase-1, SOX-10, S100, SALL4, CD138, synaptophysin, chromogranin, CD56, and Ki67 etc. may help to delineate the nature of these cells and further refine the diagnosis. Of the NETs, those with rhabdoid or plasmacytoid morphology may look similar in appearance to signet ring cell NET. All three variants demonstrate eccentrically located nuclei; however, NET with rhabdoid differentiation have densely eosinophilic intracytoplasmic globules13 and NETs with plasmacytoid differentiation have homogenous eosinophilic cytoplasm,4 while NET with signet ring differentiation show the presence of intracytoplasmic vacuole.7,10 Although NETs are generally positive for neuroendocrine markers, poor sensitivity of chromogranin has also been described in a few previously reported signet ring NETs.8,12,14
Clinically, primary hepatic NETs commonly present with abdominal pain. Clinical presentation with the features of classic carcinoid syndrome, such as skin flushing and diarrhea, is rare in primary hepatic NETs. Primary hepatic NETs usually present as slowly-growing solitary lesions (range, 1.5–27.0 cm) and remain indolent for long.15 This case presented as multiple solid and cystic lesions in both lobes of the liver, which is a rare presentation for primary hepatic NET, and the final diagnosis could only be established after biopsy.
Primary hepatic NETs generally show enhancement patterns similar to metastatic disease. The tumors appear hypointense on T1-weighted spin-echo sequences and hyperintense on T2-weighted fast spin-echo sequences on magnetic resonance imaging. Because of their hypervascular nature, contrast-enhanced CT shows intense enhancement in arterial phase with washout in portal venous and extracellular phases. 16
Surgical resection is the treatment of choice if amenable. Chemotherapy is indicated in cases of metastatic lesions. Palliative therapies can be offered in unresectable cases which include systemic 5-fluorouracil, hepatic artery embolization, and octreotide therapy.17 The disease course seems indolent for these tumors as most previously reported cases presented with slowly-growing large intrahepatic masses mostly over 5 cm.6 In general, primary hepatic NETs have been associated with survival for many years without recurrence, although deaths from metastatic disease have been reported in 18–47% of primary hepatic NETs, especially grade 2 NETs.1
To conclude, we report the first case of signet ring NET presenting as multiple solid and cystic lesions in the liver, masquerading a biliary cystadenocarcinoma on imaging. Because of their histological appearance, they pose a diagnostic dilemma with other neoplasms with signet ring cell morphology. Clinicians, radiologists and pathologists should be aware of this entity.
Notes
Ethics Statement
The ethics committee of the authors’ institution waived the need for institutional review board approval for this case report. Patient consent was obtained.
References
1. Klimstra DS. Hepatic neuroendocrine neoplasms. WHO Classification of Tumours Editorial Board. Digestive system tumours WHO classification of tumours series. 5th ed. Lyon: International Agency for Research on Cancer;2019. p. 263–264.
2. Edmondson HA. Tumors of the liver and intrahepatic bile duct. Edmondson HA, editor. Atlas of tumor pathology. 7. Washington, D.C: Armed Forces Institute of Pathology;1958. p. 105–109.
3. Dala R, Shoosmith J, Lilenbaum R, Cabello-Inchausti B. Primary hepatic neuroendocrine carcinoma: an underdiagnosed entity. Ann Diagn Pathol. 2006; 10:28–31.
4. Xue Y, Reid MD, Pehlivanoglu B, Obeng RC, Jiang H, Memis B, et al. Morphologic variants of pancreatic neuroendocrine tumors: clinicopathologic analysis and prognostic stratification. Endocr Pathol. 2020; 31:239–253.
5. Xu C, Li HX, Zhu Y, Pan BJ, Zhang ZH. Gastric signet ring cell neuroendocrine tumor: report of a case. Zhonghua Bing Li Xue Za Zhi. 2020; 49:941–943.
6. Oh YH, Kang GH, Kim OJ. Primary hepatic carcinoid tumor with a paranuclear clear zone: a case report. J Korean Med Sci. 1998; 13:317–320.
7. Aoki K, Sakamoto M, Mukai K, Kosuge T, Takayama T, Hiroshashi S. Signet-ring cell carcinoid: a primary hepatic carcinoid tumor with cytoplasmic inclusions comprising of aggregates of keratin. Jpn J Clin Oncol. 1992; 22:54–59.
8. Haq S, Batra VV, Majumdar K, Javed A, Agarwal AK, Sakhuja P. Signet ring cell neuroendocrine tumor liver with mesenteric metastasis: description of a rare phenomenon, with literature review. J Cancer Res Ther. 2015; 11:658.
9. Madakshira MG, Radotra BD, Singh V. Signet ring: a rare morphology of metastatic neuroendocrine tumor. Indian J Pathol Microbiol. 2019; 62:335–336.
10. Sioutos N, Virta S, Kessimian N. Primary hepatic carcinoid tumor. An electron microscopic and immunohistochemical study. Am J Clin Pathol. 1991; 95:172–175.
11. Zhu H, Sun K, Ward SC, Schwartz M, Thung SN, Qin L. Primary hepatic signet ring cell neuroendocrine tumor: a case report with literature review. Semin Liver Dis. 2010; 30:422–428.
12. Sabbah M, Trad D, Bellil N, Jouini R, Ouakaa A, Elloumi H, et al. Signet ring cell neuroendocrine tumor. Radiol Diagn Imaging. 2019; 3:1–2.
13. Miyazaki T, Aishima S, Fujino M, Ozono K, Kubo Y, Ushijima Y, et al. Neuroendocrine tumor of the pancreas with rhabdoid feature. Virchows Arch. 2018; 473:247–252.
14. Gut P, Czarnywojtek A, Fischbach J, Bączyk M, Ziemnicka K, Wrotkowska E, et al. Chromogranin A-unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016; 12:1–9.
15. Quartey B. Primary hepatic neuroendocrine tumor: what do we know now? World J Oncol. 2011; 2:209–216.
16. van der Hoef M, Crook DW, Marincek B, Weishaupt D. Primary neuroendocrine tumors of the liver: MRI features in two cases. Abdom Imaging. 2004; 29:77–81.
17. Wängberg B, Nilsson O, Johanson VV, Kölby L, Forssell-Aronsson E, Andersson P, et al. Somatostatin receptors in the diagnosis and therapy of neuroendocrine tumor. Oncologist. 1997; 2:50–58.
Figure 1
Contrast-enhanced computed tomography findings. There are cystic lesions in both lobes of liver with peripheral solid component with heterogeneous enhancement on arterial phase (A). On venous phase images, the solid components remain heterogeneous (B). Some of the solid components show calcification (C).
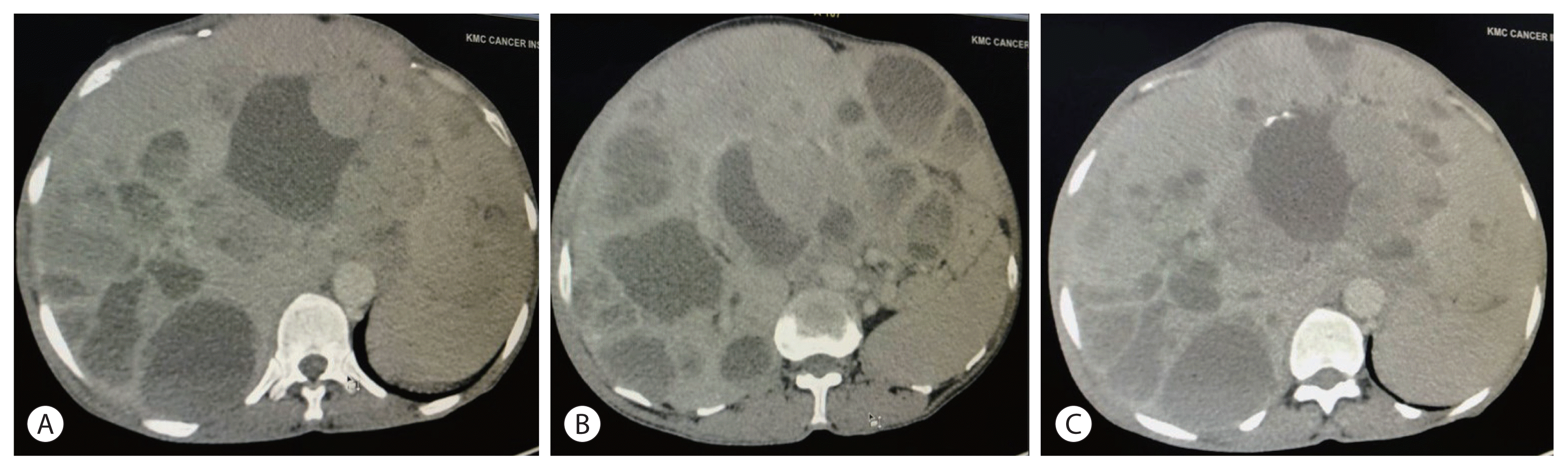
Figure 2
Positron emission tomography computed tomography shows multiple liver lesions with uptake in the solid components (A), and intraabdominal lymph nodes (B).
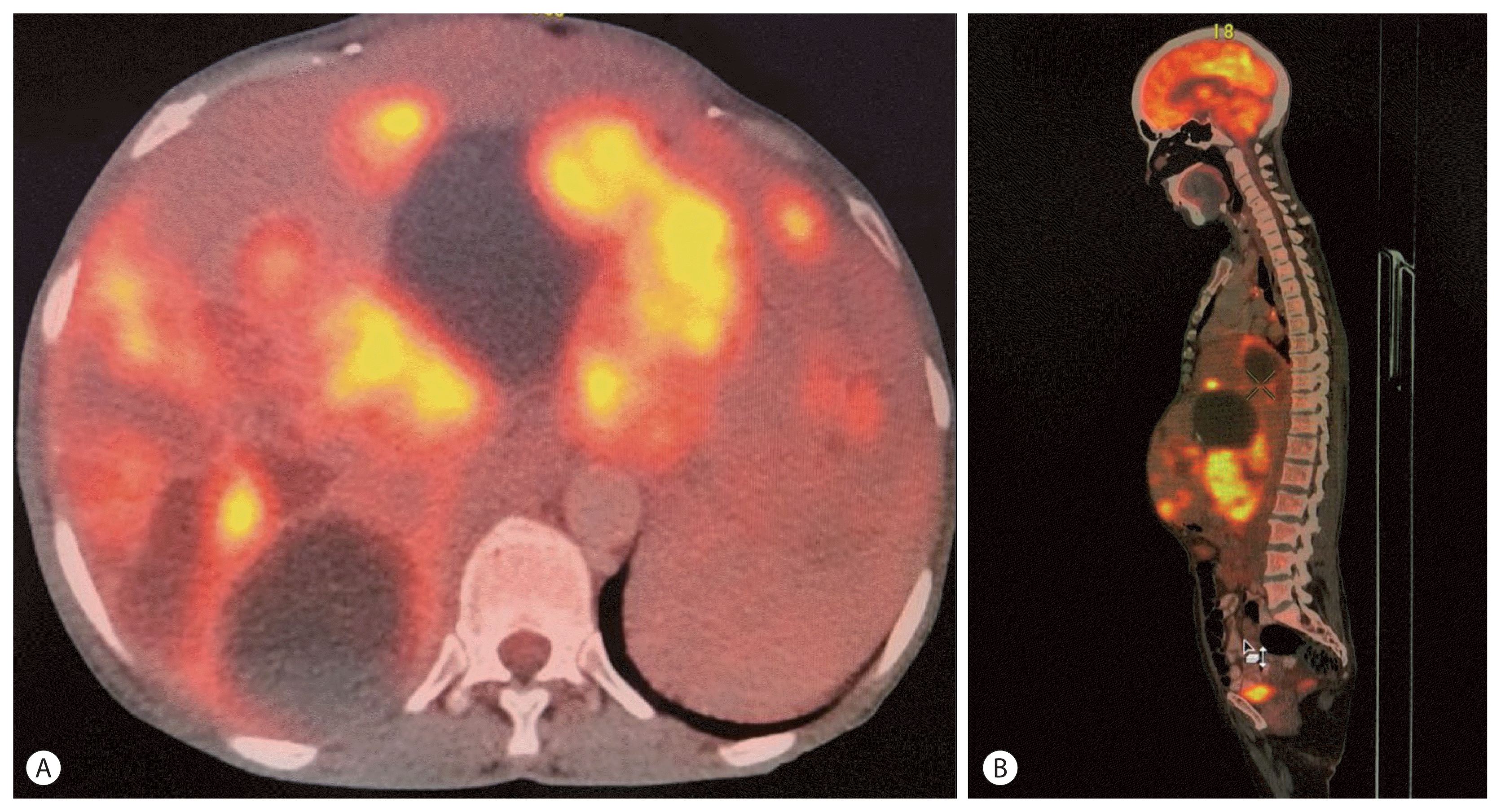
Figure 3
Microscopic features of the tumor. Low power magnification demonstrates fragments of tumor along with the non-neoplastic hepatic parenchyme (A). The tumor demonstrates a papillary architecture, with the tumor cells centered around microvasculature (B). High power magnification demonstrates the details of the tumor cells (C): the tumor cells demonstrate eccentrically located nuclei and intracytoplasmic vacuoles with a signet ring cell-like appearance. (A) Hematoxylin-eosin, original magnification ×100, (B) ×200, (C) ×400. Mucicarmine stain demonstrates the absence of mucin in the cytoplasm (D) (original magnification, ×400).
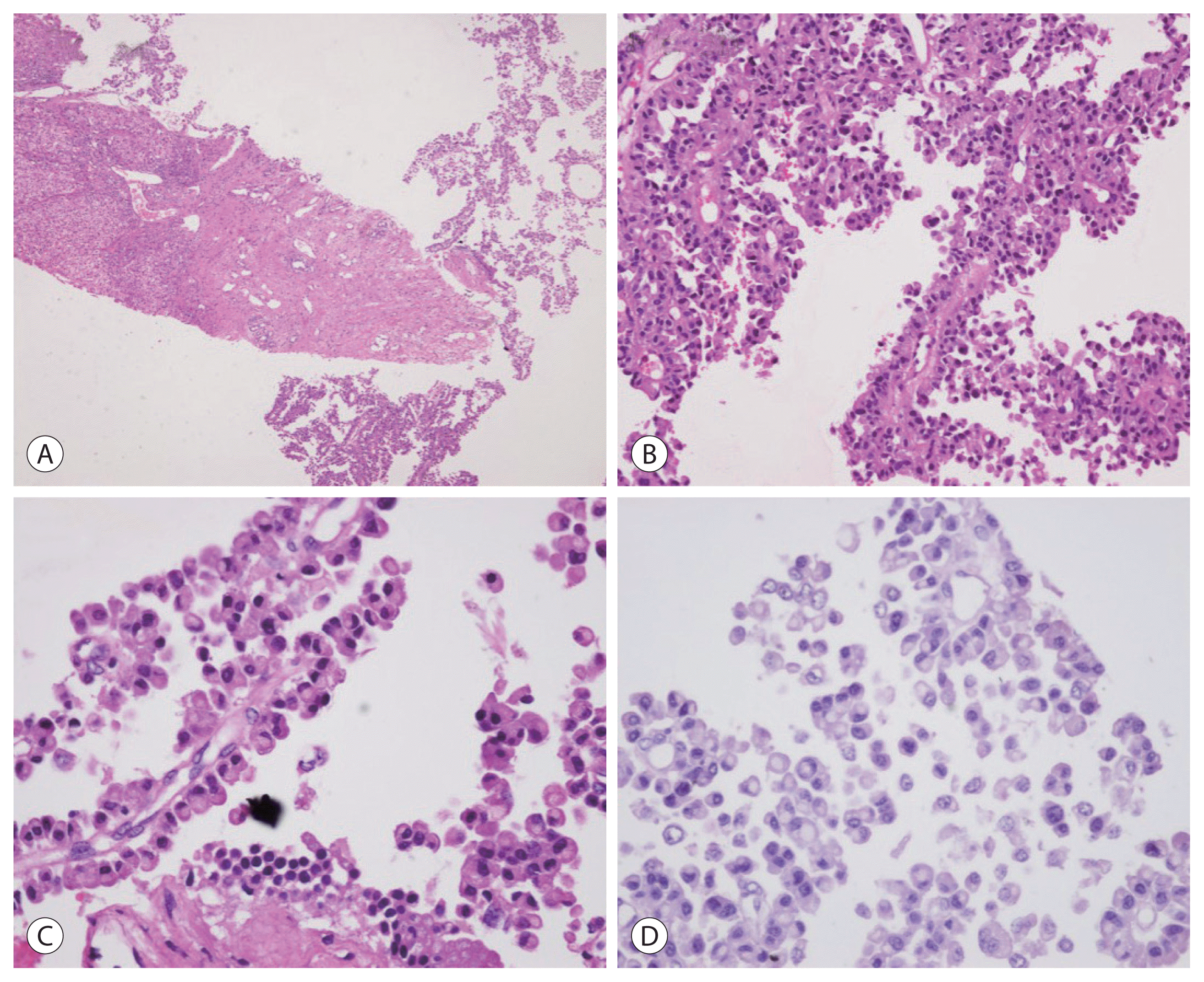
Figure 4
Immunohistochemical stain results of the tumor. The tumor cells express pan-cytokeratin (A), synaptophysin (C), CD56 (D), chromogranin A (E) and are negative for thyroid transcription factor-1 (B). The Ki-67 labeling index was 2–3% (F). (original magnification x100 [A–D], x200 [E], x400 [F]).
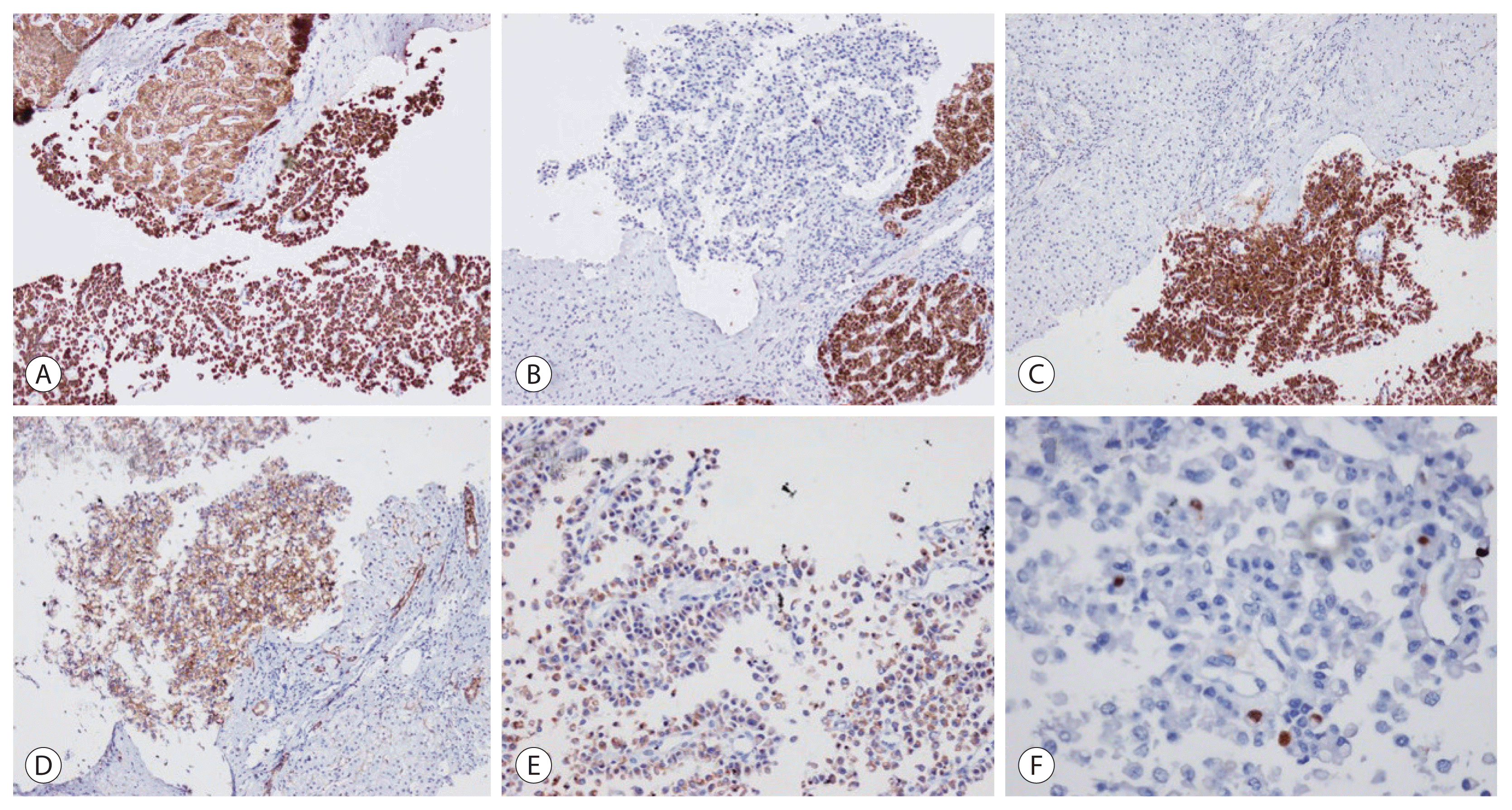
Table 1
Summary of the literature on primary signet ring neuroendocrine tumors of the liver
| Authors | Reference | Country | Year | Age (years)/Sex | Clinical features | Gross features | Histologic findings | Treatment | Follow-up | Metastasis |
|---|---|---|---|---|---|---|---|---|---|---|
| Sioutos et al. | 10 | USA | 1991 | 68/M | 8 months, enlarging mass | 11 cm, well demarcated | Nests and trabeculae with intracytoplasmic vacuoles | Resection | 49 months NED | Nil |
| Aoki et al. | 7 | Japan | 1992 | 39/F | 6 months, hypochondrial mass | 10 cm, partially encapsulated with cystic degeneration | Nests and trabeculae with intracytoplasmic vacuoles | Resection | 14 months NED | Nil |
| Oh et al. | 6 | Korea | 1998 | 48/M | 8 years, abdominal fullness | 13 cm, well demarcated with areas of hemorrhage and hyaline degeneration | Nest, strands, ribbons separated by delicate fibrovascular septae | Resection | 5 months NED | Nil |
| Zhu et al. | 11 | USA | 2010 | 49/M | 4 years, incidental finding | 2.7 cm, well demarcated | Sheets of monotonous epithelioid cells surrounded by delicate vascular channels | Resection | 10 months NED | Nil |
| Haq et al. | 8 | India | 2015 | 43/F | 2 years, low grade fever and abdominal pain | 10.5 cm, mass in right lobe | Sheets and nests separated by fibrocollagenous tissue | 3 cycles of chemotherapy: dacarbazine, etoposide, ifosfamide, carboplatin | 18 months well preserved | Nil |
| Bansal et al. | Current case | India | 2021 | 47/F | Abdominal pain | 10.2 cm, multiple cystic lesions in both liver lobes | Papillary and nested pattern | 3 cycles of chemotherapy: carboplatin, doxorubicin, etoposide | 14 months stable disease | Lymph nodes |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download