Abstract
JX-594 is a modified oncolytic poxvirus designed to selectively replicate in and destroy cancer cells. In a pilot study, JX-594 injection followed by sorafenib was well-tolerated in three patients and associated with objective tumor responses. In this study, we report a case in which a patient with advanced hepatocellular carcinoma and portal vein thrombosis was treated with a combination of JX-594 and sorafenib.
In South Korea, hepatocellular carcinoma (HCC) is the sixth most common cause of cancer.1 The development of imaging modalities has enabled more accurate and earlier diagnosis of HCC, but the prognosis of advanced HCC remains poor. Unfortunately, in advanced HCC, the role of both locoregional therapy and systemic chemotherapy is limited. Advanced HCC patients with preserved liver function treated with sorafenib had a median overall survival of 6.5–10.7 months.2
The idea of using live viruses to treat cancer has been studied since reports of tumor regression following viral infection were published.3 Live viruses, which can selectively replicate in cancer cells and induce cell lysis, were first engineered in the 1990s. However, the prototype oncolytic viruses showed weak potency and unfavorable clinical outcomes.4 To increase potency, poxviruses such as vaccinia have been modified as oncolytic viruses.5
JX-594 is a modified oncolytic poxvirus designed to selectively replicate in and destroy cancer cells. JX-594 was not only well-tolerated by patients with HCC in phase 1 trial but also induced replication within tumor, tumor necrosis and decreased tumor perfusion.6 Since JX-594 has a different mode of action compared to sorafenib, which is an inhibitor of B-raf and vascular endothelial growth factor receptor, combination of these two agents has a chance to increase efficacy in oncolysis. The combination therapy was treated to three HCC patients in a pilot study. Since sorafenib inhibited the replication of JX-594 in vitro, sequential therapy was administered instead of concurrent treatment of the two agents. The sequential combination therapy was well-tolerated by the three patients and associated with objective tumor responses.7 A randomized phase 3 study was initiated in October 2015 to determine whether treatment with JX-594 followed by sorafenib increases survival compared to treatment with sorafenib in patients with advanced HCC who have not received prior systemic therapy. In the following case report, one patient with aggravated HCC and newly developed portal vein thrombosis (PVT) despite receiving transarterial chemoembolization (TACE) undergoes treatment with JX-594, an engineered oncolytic virus, followed by sorafenib.
A 75-year-old man with advanced HCC, which showed no response to TACE, was admitted to the hospital for a therapeutic trial. He was a 90-pack-year ex-smoker and heavy drinker. He had a history of alcoholic liver cirrhosis, hypertension, diabetes mellitus, and hyperlipidemia. He had no familial history of malignant tumors. TACE was administered three times in the past nine months, but the tumor size increased and newly appearing thrombosis in the middle hepatic vein was noted in a follow-up computed tomography (CT) scan (Fig. 1). The patient was diagnosed with the International Union for Cancer Control cancer stage T3N0M0, and Barcelona Clinic Liver Cancer stage C. Laboratory findings reported normal complete blood count, slightly elevated serum serum aspartate aminotransferase (AST) 43 IU/L, normal serum alanine transferse (ALT) 13 IU/L elevated serum creatinine (1.4 mg/dL), normal sodium level (142 mmol/L) and normal albumin level, bilirubin and prothrombin time. The patient’s serum alpha-fetoprotein (AFP) level was 30,110 ng/mL. His Child-Pugh score was 5A and Model for End-Stage Liver Disease was 11.
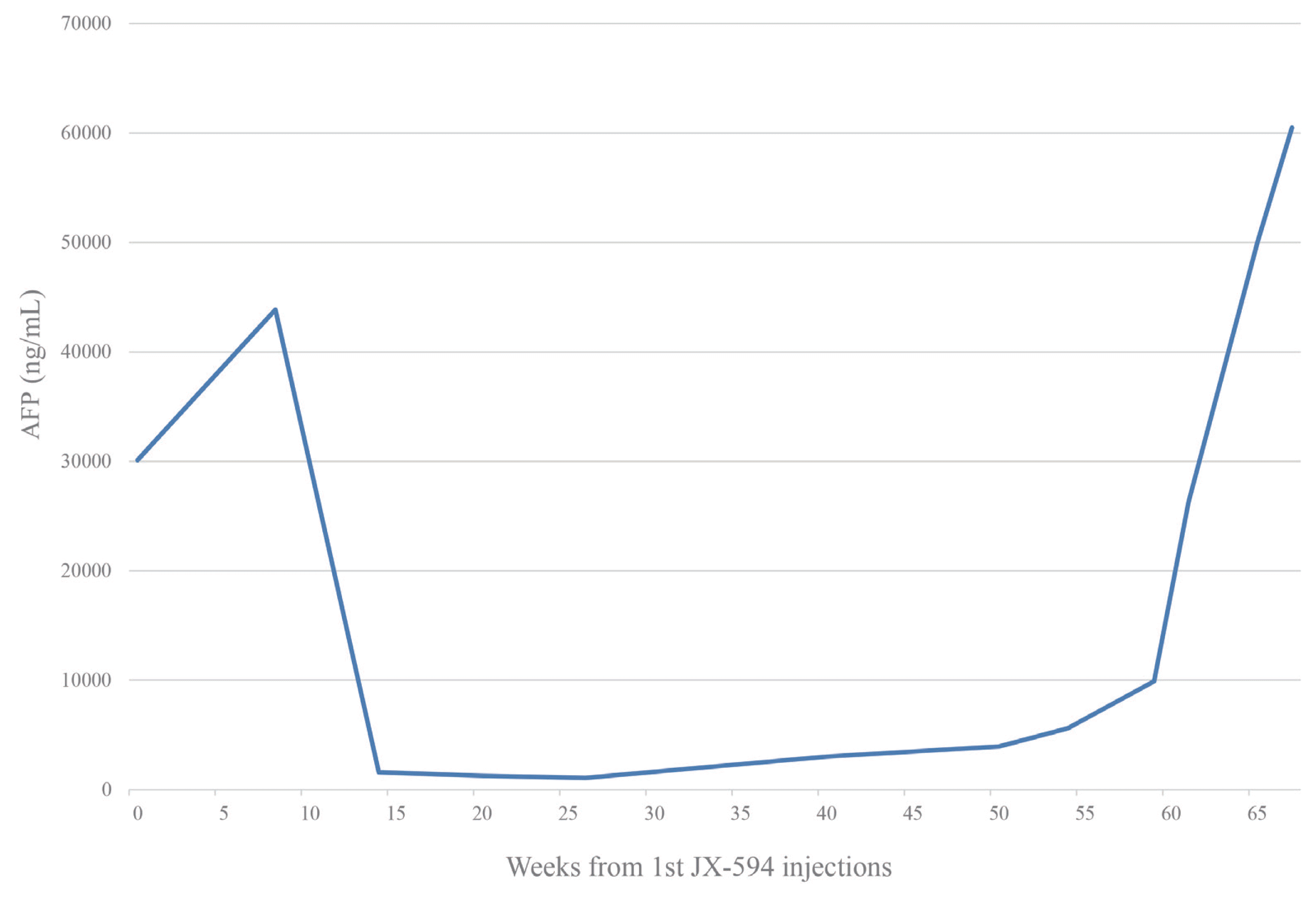
He was a candidate for systemic chemotherapy and enrolled in an open-label trial of JX-594, an oncolytic virus, combined with sorafenib. After thorough review and upon obtaining informed consent from the patient, JX-594 was injected directly into the tumor via ultrasound guidance in three trials administered in three-week intervals, at a dose of 1×109 plaque forming units per dose. The procedure was performed by a professionally trained radiologist. Initially, the JX-594 injections were tolerable to the patient. The only adverse effect observed was transient flu-like symptoms such as mild fever and mild myalgia. Sorafenib was given as combination chemotherapy at 400 mg twice daily, starting three weeks after the JX-594 injection. A CT scan 1.5 months after the oncolytic virus injection showed increased tumor size, but the arterial enhancing portion of the tumor was decreased (Fig. 2). The AFP level was also decreased to 1,576 ng/mL (Fig. 3). The overall response evaluation was a partial response (PR) by the modified Response Evaluation Criteria in Solid Tumors (mRECIST).
During the next four months, the total size of the tumor continued to decrease, and finally reached 64% of the baseline at week 16 of the study. During that period, the base tumor structure collapsed and only a few small enhancing nodules remained (Fig. 4A). According to the mRECIST criteria, the tumor response was PR, and the patient continued receiving sorafenib treatment. From week 16 to week 26, the tumor size measured in CT imaging showed no interval change, but the small nodules persisted. The tumor response showed stable disease. However, in week 30 of the study, the size of the small enhancing nodules increased (Fig. 4B). The diameter of the whole tumor showed no significant change. Between weeks 36 and 40, the patient was admitted for eight days with diarrhea and fever. He continued to take sorafenib for three weeks. After recovering from diarrhea, the week 40 CT scan showed an increase in the tumor size from 4.0 cm to 4.6 cm in the largest diameter (Fig. 4C). The AFP level also increased to 3,013 ng/mL. Despite the clinical progression of the disease, there were no other effective treatments and the patient continued on sorafenib. However, he experienced diarrhea, general weakness, and loss of appetite. His dose of sorafenib was decreased to half. He stopped sorafenib after week 54 as multiple, new intrahepatic lesions appeared on the CT images and his performance status deteriorated (Fig. 4D). From week 54, the goal of treatment was changed to best supportive care. He expired from sudden respiratory arrest on week 67 after an injection of JX-594. This study was approved by the Institutional Review Board of Soonchunhyang University Hospital, Bucheon, Korea (SCHBC-2015-12-003). The study protocol conformed to ethical guidelines of the World Medical Association Declaration of Helsinki.
Oncolytic viruses have multiple mechanisms of action.8 First, JX-594 is a modified poxvirus designed to selectively replicate in cancer cells.9 It can selectively infect and directly destroy tumor cells. The thymidine kinase (TK) gene of JX-594 is inactivated, so viral replication becomes dependent upon cellular TK, which is more concentrated in tumor cells with abnormal cell cycles than in normal cells. Infection and replication result in an acute inflammatory response, which is toxic to tumor cells. Second, JX-594 induces an adaptive immune response. A transgene encoding human-granulocyte-macrophage colony-stimulating factor (GM-CSF) was inserted to induce antitumor immunity. In addition to producing viral antigen, the modified virus can release GM-CSF to induce an acute inflammatory response. T-cell activation then follows to establish adaptive immunity. Third, JX-594 has acute anti-vascular effects. A group of HCC patients who received intra-tumoral JX-594 injections in a phase II study showed decreased tumor perfusion measured by dynamic magnetic imaging five days after JX-594 injection. Normal liver tissues did not show reduced perfusion.10
Oncolytic virus injections directly to the tumor itself were both tolerable and safe. JX-594 treatment was well-tolerated in phase I and showed promising efficacy in a phase II trial.6,11 Transient flu-like symptoms were the only adverse effect. There was no acute increase in AST or ALT, indicating there was minimal toxicity on healthy hepatocytes. Adverse effects on blood vessels, such as the occurrence of hypertension, were also not found. No new adverse effects related to the oncolytic virus were observed in this patient.
Advanced HCC patients with preserved liver function and treated with sorafenib had a median overall survival of 6.5–10.7 months.2 However, advanced HCC patients with portal vein thrombosis and treated with sorafenib showed an even shorter median overall survival of 3.1–7.2 months.12,13 In this case, the patient had HCC with portal vein thrombosis when initial dosage of JX-594 was injected and survived 15.6 months after the first injection. It could be suggested that combination of JX-594 with sorafenib induced survival gain of 8.4 to 12.5 months, which is longer than that of sorafenib alone. Therefore, restricted to this case, it could be suggested that JX-594 had more dominant effect on the patient’s survival gain.
The best response in this case was obtained four months after the injection and was sustained for three more months. It is clear that the oncolytic virus demonstrated an antitumor effect at the beginning. However, the final tumor response was unfavorable in this case. It is uncertain whether the virus weakened or the host immunity interfered with the virus. Other trials have reported that neutralizing antibodies to JX-594 did not interfere with intra-tumoral JX-594.6,14 However, there is a possibility that the host immune environment may change and attack the virus with time. The cause of failure should be investigated by future studies.
The interim results suggested that the study was unlikely to meet the primary objective by the time of the final analysis and the enrollment of the patient stopped in August 2019. The data of phase 3 trial of JX-594 is not officially released yet. Other multiple systemic agents have been studied to improve outcomes. Novel chemotherapeutics, such as lenvatinib, regorafenib and nivolumab, have undergone clinical trial, but the outcomes of these new agents show no significant difference compared to sorafenib.15–17 Recently, atezolizumab plus bevacizumab has proven better outcomes in overall survival and progression-free survival than sorafenib in a global, open-label, phase 3 randomized trial named IM-brave150. Atezolizumab-bevacizumab resulted in progression-free survival of 6.8 months, 2.5 months more than sorafenib.18 This trial included patients who had portal vein invasion, and is still ongoing. We reported a case of sequential combination therapy with oncolytic virus and sorafenib. The patient obtained a favorable response in the first seven months, but the tumor progressed thereafter. Further research is needed to analyze the mechanism of progression and enhance the efficacy of oncolytic virus injection.
REFERENCES
1. Kweon SS. Updates on cancer epidemiology in Korea, 2018. Chonnam Med J. 2018; 54:90–100.
2. Hsu CH, Shen YC, Shao YY, Hsu C, Cheng AL. Sorafenib in advanced hepatocellular carcinoma: current status and future perspectives. J Hepatocell Carcinoma. 2014; 1:85–99.
3. Huebner RJ, Rowe WP, Schatten WE, Smith RR, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956; 9:1211–1218.
4. Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007; 67:429–432.
5. Thorne SH, Hwang TH, O’Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007; 117:3350–3358.
6. Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008; 9:533–542.
7. Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011; 19:1170–1179.
8. Breitbach CJ, Bell JC, Hwang TH, Kirn DH, Burke J. The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother. 2015; 4:25–31.
9. Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, Ilkow C, et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther. 2012; 20:749–758.
10. Breitbach CJ, Arulanandam R, De Silva N, Thorne SH, Patt R, Daneshmand M, et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013; 73:1265–1275.
11. Breitbach CJ, Moon A, Burke J, Hwang TH, Kirn DH. A phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol Biol. 2015; 1317:343–357.
12. Jeong SW, Jang JY, Shim KY, Lee SH, Kim SG, Cha SW, et al. Practical effect of sorafenib monotherapy on advanced hepatocellular carcinoma and portal vein tumor thrombosis. Gut Liver. 2013; 7:696–703.
13. Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018; 82:469–478.
14. Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008; 16:1637–1642.
15. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018; 391:1163–1173.
16. Personeni N, Pressiani T, Santoro A, Rimassa L. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs Context. 2018; 7:212533.
17. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. LBA38_PR - CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019; 30:v874–v875.
18. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Figure 1
(A) After three sessions of transarterial chemoembolization, the tumor size increased to 5.8 cm in diameter. (B) Tumor thrombus was noted in the middle hepatic vein (arrow).
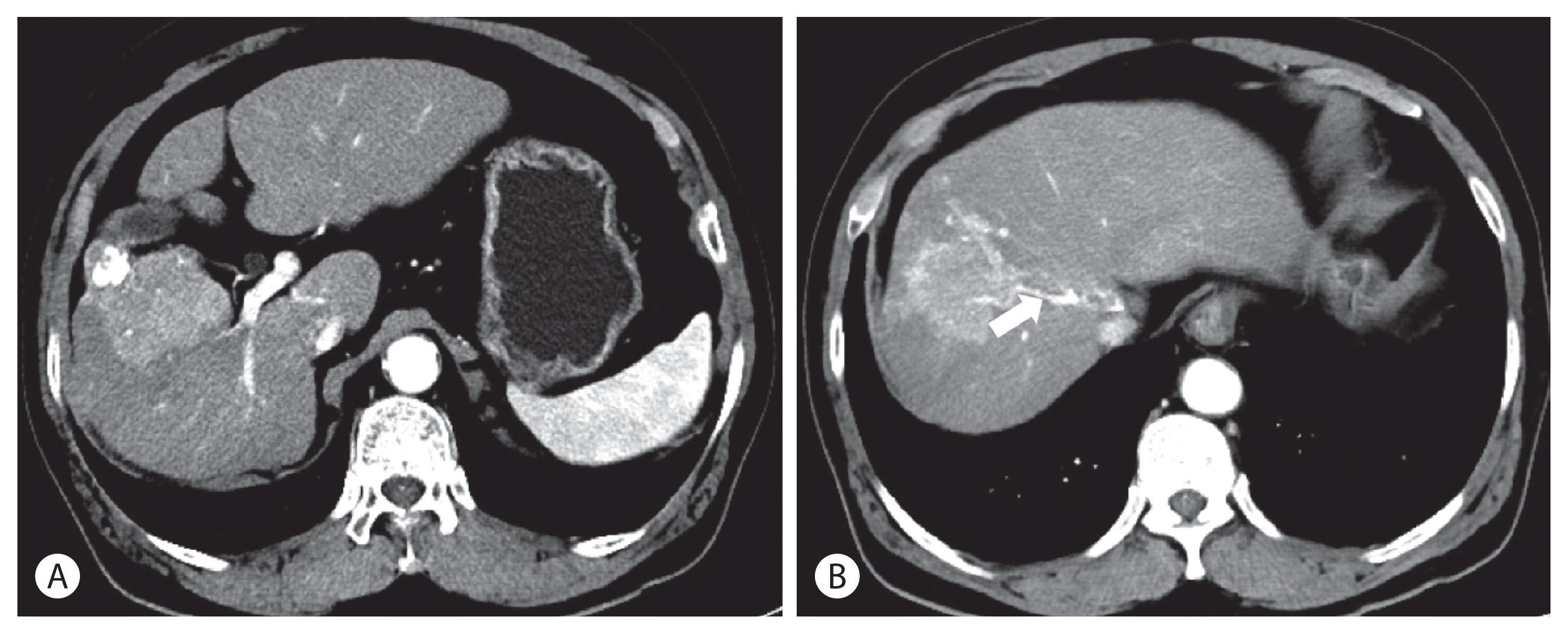
Figure 2
Study week 6 of JX-594 injections. The size of the total tumor increased from 5.8 cm to 7.0 cm in diameter, but the enhancing portion of the tumor decreased.
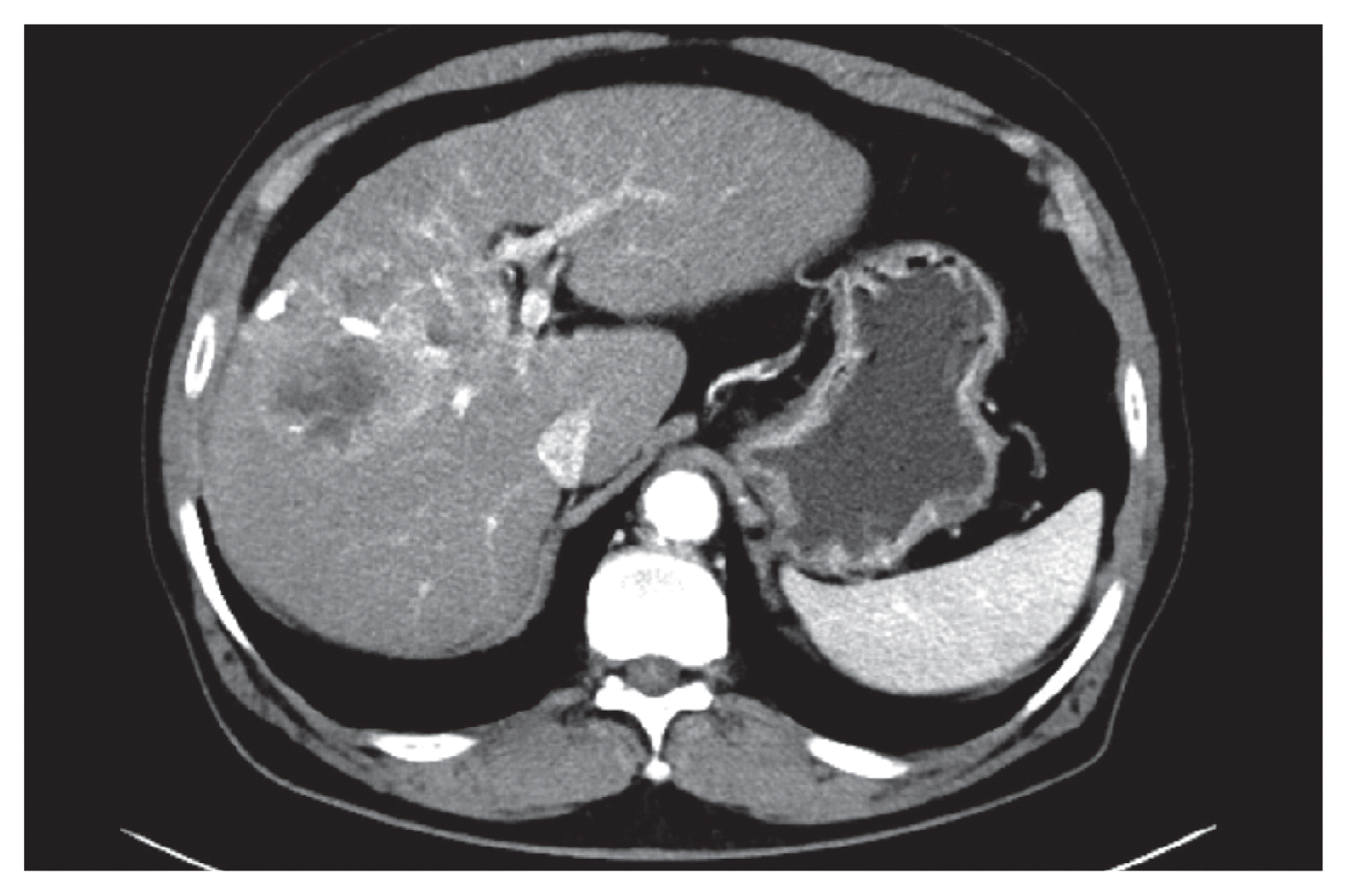
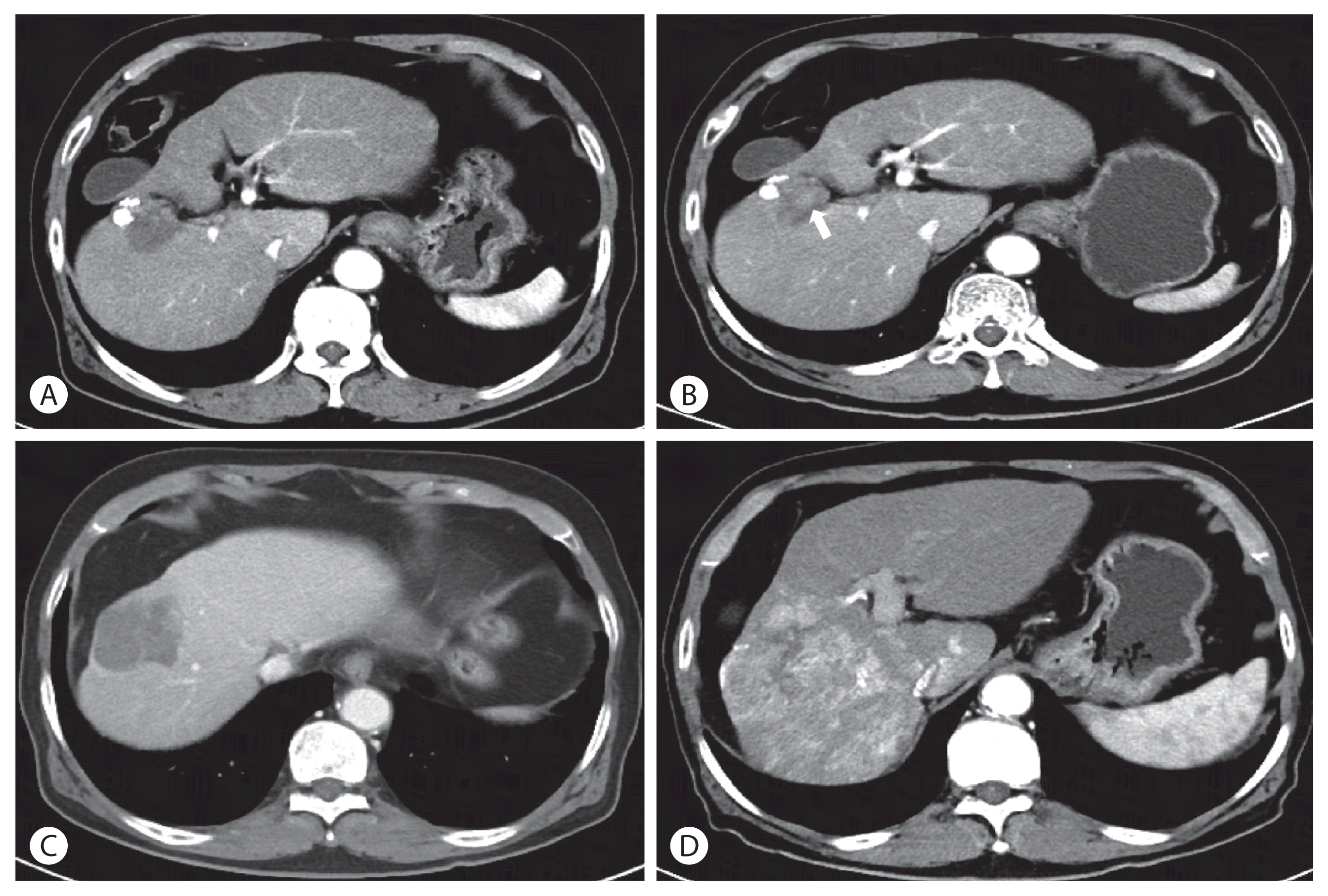
Figure 4
(A) Study week 16 of JX-594 injections. The total size of the tumor decreased to 3.7 cm in diameter. There are only a few small arterial enhancing nodules inside the tumor. (B) Study week 30 of JX-594 injections. The size of the enhancing nodules within the necrotic material (arrow) increased. (C) Study week 40 of JX-594 injections. The total tumor size increased to 4.6 cm in diameter. (D) Study week 54 of JX-594 injections. Multiple intrahepatic metastases appeared.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download